Abstract
Background
Corneal neovascularization (NV) plays an important role in the pathogenesis of corneal disorders. Recently, triamcinolone acetonide (TA) has been reported as a potential treatment for ocular angiogenesis. However, there are no reports on the inhibitory effect of TA on the corneal NV.
Methods
Triamcinolone acetonide (2 mg) was administered to four rabbits' eyes by a subconjunctival injection immediately after a basic fibroblast growth factor (bFGF)-pellet was placed into the cornea. As a control, four eyes received an injection of distilled water. Four weeks later, the inhibition of corneal NV was evaluated as the percentage ratio of the vessel invasion area to the area that was sandwiched between the pellet and the limbus cornea. To identify the characteristic appearance of new corneal vessels, the control cornea was examined by using the antibody of vascular endothelial growth factor (VEGF). To confirm TA concentration in TA-treated corneas, the TA level was measured using high-performance liquid chromatography.
Results
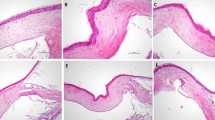
Neovascularization from the limbus to the pellet was detected in control eyes 4 weeks after the bFGF pellet implantation. TA-treated eyes demonstrated the inhibition of the neovascular response to the pellet. The severity of NV as compared between control and TA-treated eyes was statistically significant (P<0.05). Morphologically, new vessel growth was shown in the control cornea, and endothelial cells of new vessels were positively stained with the antibody of VEGF. TA concentration in TA-treated corneas at 2 weeks showed 63.5±42.8 μg/g (n=4, mean ± SD), while TA was not detected in control and TA-treated corneas at 4 weeks. The level of TA was effectively maintained for at least 2 weeks after the subconjunctival injection.
Conclusion
We have demonstrated that subconjunctival TA administration inhibited rabbit corneal NV. This agent may prove useful in the treatment of corneal angiogenic disorders.



Similar content being viewed by others
References
Avery RL, Connor TB, Farazdaghi M (1990) Systemic amiloride inhibits experimentally induced neovascularization. Arch Ophthalmol 108:1474–1478
Blei F, Wilson EL, Mignatti P (1993) Mechanism of action of angiostatic steroids: suppression of plasminogen activator activity via stimulation of plasminogen activator inhibitor synthesis. J Cell Physiol 155:568–578
Challa JK, Gillies MC, Penfold PL (2000) Exudative macular degeneration and intravitreal triamcinolone: 18 month follow up. Aust N Z J Ophthalmol 26:277–281
Ciulla TA, Criswell MH, Danis RP (2001) Intravitreal triamcinolone acetonide inhibits choroidal neovascularization in a laser-treated rat model. Arch Ophthalmol 119:399–404
Cockerill GW, Gamble JR, Vadas MA (1995) Angiogenesis: models and modulators. Int Rev Cytol 159:113–160
Danis RP, Bingaman DP, Yang Y (1997) Inhibition of preretinal and optic nerve head neovascularization in pigs by intravitreal triamcinolone acetonide. Ophthalmology 103:2099–2104
Danis RP, Ciulla TA, Pratt LM (2000) Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. Retina 20:244–250
Dastgheib K, Green WR (1994) Granulomatous reaction to Bruch's membrane in age-related macular degeneration. Arch Ophthalmol 112:813–818
Deutsch TA, Hughes WF (1979) Suppressive effects of indomethacin on thermally induced neovascularization of rabbit corneas. Am J Ophthalmol 87:536–540
Duffin RM, Weissman BA, Glasser DB (1997) Flurbiprofen in the treatment of corneal neovascularization induced by contact lenses. Am J Ophthalmol 93:607–611
Folkman J, Ingber DE (1987) Angiostatic steroids. Method of discovery and mechanism of action. Ann Surg 206:374–383
Folkman J, Weisz PB, Joullie MM (1989) Control of angiogenesis with synthetic heparin substitutes. Science 243:1490–1495
Gillies MC, Simpson JM, Luo W (2003) A randomized clinical trial of a single dose of intravitreal triamcinolone acetonide for neovascular age-related macular degeneration: one-year results. Arch Ophthalmol 121:667–673
Gimbrone MA, Cotran RS, Leapman SB (1974) Tumor growth and neovascularization: an experimental model using the rabbit cornea. J Natl Cancer Inst 52:413–427
Inoue M, Takeda K, Morita K (2004) Vitreous concentrations of triamcinolone acetonide in human eyes after intravitreal or subtenon injection. Am J Ophthalmol 138:1046–1048
Ip MS, Kumar KS (2002) Intravitreous triamcinolone acetonide as treatment for macular edema from central retinal vein occlusion. Arch Ophthalmol 120:1217–1219
Ishibashi T, Miki K, Sorgente N (1985) Effects of intravitreal administration of steroids on experimental subretinal neovascularization in the subhuman primate. Arch Ophthalmol 103:708–711
Jonas JB, Kreissig I, Degenring RF (2003) Intravitreal triamcinolone acetonide for pseudophakic cystoid macular edema. Am J Ophthalmol 136:384–386
Killingsworth MC, Sarks JP, Sarks SH (1990) Macrophages related to Bruch's membrane in age-related macular degeneration. Eye 4:613–621
Kvanta A, Algvere PV, Berglin L (1996) Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Investig Ophthalmol Vis Sci 37:1929–1934
Loughman MS, Chatzistefanou K, Gonzalez EM (1997) Experimental corneal neovascularisation using sucralfate and basic fibroblast growth factor. Aust N Z J Ophthalmol 24:289–295
Murata M, Nakagawa M, Takahashi S (1997) Inhibitory effects of plasminogen fragment on experimentally induced neovascularization of rat corneas. Graefe Arch Clin Exp Ophthalmol 235:584–586
Murata M , Kador PF, Sato S (2000) Vascular endothelial growth factor (VEGF) enhances the expression of receptors and activates mitogen-activated protein (MAP) kinase of dog retinal capillary endothelial cells. J Ocular Pharmacol Ther 16:383–391
Oh H, Takagi H, Takagi C, Suzuma K, Otani A, Ishida K, Matsumura M, Ogura Y, Honda Y (1999) The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Investig Ophthalmol Vis Sci 40:1891–1898
Penfold PL, Gyory JF, Hunyor AB (1995) Exudative macular degeneration and intravitreal triamcinolone. A pilot study. Aust N Z J Ophthalmol 23:293–298
Peyman GA, Cheema R, Conway MD (2000) Triamcinolone acetonide as an aid to visualization of the vitreous and the posterior hyaloid during pars plana vitrectomy. Retina 20:554–555
Shweiki D, Itin A, Soffer D, Keshet E (1992) Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359:843–845
Sonoda KH, Enaida H, Ueno A (2003) Pars plana vitrectomy assisted by triamcinolone acetonide for refractory uveitis: a case series study. Br J Ophthalmol 87:1010–1014
Tabata Y, Yamada K, Miyamoto S (1998) Bone regeneration by basic fibroblast growth factor complexed with biodegradable hydrogels. Biomaterials 19:807–815
Wolff JE, Guerin C, Laterra J (1993) Dexamethasone reduces vascular density and plasminogen activator activity in 9 L rat brain tumors. Brain Res 604:79–85
Yang CF, Yasukawa T, Kimura H (2000) Experimental corneal neovascularization by basic fibroblast growth factor incorporated into gelatin hydrogel. Ophthalmic Res 32:19–24
Author information
Authors and Affiliations
Corresponding author
Additional information
No human subjects are involved as experimental animals were used in this study
Rights and permissions
About this article
Cite this article
Murata, M., Shimizu, S., Horiuchi, S. et al. Inhibitory effect of triamcinolone acetonide on corneal neovascularization. Graefe's Arch Clin Exp Ophthalmo 244, 205–209 (2006). https://doi.org/10.1007/s00417-005-0036-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-005-0036-1