Abstract
Background
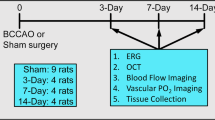
Ocular ischemic syndrome is a devastating eye disease caused by severe carotid artery stenosis. The reduction of blood flow produced by bilateral common carotid artery occlusion (BCCAO) of rats for 7 days induces events related to gliosis with no evident histological damage. However, retinal degeneration and cellular death occur after 90 days of BCCAO. Our purpose has been to investigate the effects of BCCAO for 30 days in the retina of adult rats.
Methods
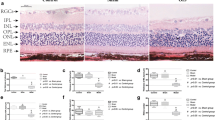
Adult Wistar rats were submitted to BCCAO or sham surgery. Both direct and consensual pupillary light reflexes were investigated before and after surgery, everyday for the first week and weekly for 30 days. After 1 month, eyes were enucleated and embedded in paraffin. The retinal ganglion cell (RGC) density and thickness of the internal plexiform (IPL), internal nuclear, outer plexiform, and outer nuclear layers were estimated.
Results
Four rats of the BCCAO group (50%) lost the direct pupillary reflex in both eyes, three rats (37%) lost this reflex in one eye, and only one (13%) maintained it in both eyes. RGC density (cells/mm) was diminished in the BCCAO group, and a significant decrease was found in the total retina and IPL thickness; however, no changes were evident in the other layers. BCCAO pupillary-reflex-negative rats presented with a significant decrease in total retinal thickness and retinal ganglion cell density compared with the sham group. Both BCCAO pupillary-reflex-positive) and -negative rats showed a decrease in IPL compared with the sham group.
Conclusion
This study demonstrates that BCCAO for 30 days induces functional and morphological damage to the retina with loss of the pupillary reflex and a decrease in IPL thickness and RGC number. We suggest that this protocol might be used as a model for ocular ischemic syndrome in the rat.




Similar content being viewed by others
References
Adachi K, Kashii S, Masai H, Ueda M, Kaneda K, Kume T, Akaike A, Honda Y (1998) Mechanism of the pathogenesis of glutamate neurotoxicity in retinal ischemia. Graefe Arch Clin Exp Ophthalmol 236:766–774
Arteni NS, Salgueiro J, Torres I, Achaval M, Netto CA (2003) Neonatal cerebral hypoxia-ischemia causes lateralized memory impairments in the adult rat. Brain Res 973:171–178
Bachman JA (1995) Exacerbation of ocular ischemic syndrome following cataract surgery. Clin Eye Vis Care 7:139–142
Barnett NL, Osborne NN (1995) Prolonged bilateral carotid artery occlusion induces electrophysiological and immunohistochemical changes to the rat retina without causing histological damage. Exp Eye Res 61:83–90
Beti NF, Mateo I, La Calle VD, Ruiz J, Beldarrain MG, Garcia-Monco JC (2003) The ocular ischemic syndrome. Clin Neurol Neurosurg 106:60–62
Costa VP, Kuzniec S, Molnar LJ, Cerri GC, Puech-Leao P, Carvalho CA (1997) Clinical findings and hemodynamic changes associated with severe occlusive carotid artery disease. Ophthalmology 104:1994–2002
Davidson CM, Pappas BA, Stevens WD, Fortin T, Bennett SA (2000) Chronic cerebral hypoperfusion: loss of pupillary reflex, visual impairment and retinal neurodegeneration. Brain Res 859:96–103
Drago F, Valzelli S, Emmi I, Marino A, Scalia CC, Marino V (2001) Latanoprost exerts neuroprotective activity in vitro and in vivo. Exp Eye Res 72:479–486
Dugan JD Jr, Green WR (1991) Ophthalmologic manifestations of carotid occlusive disease. Eye 5:226–238
Jacobs NA, Ridway AEA (1985) Syndrome of ischaemic ocular inflammation: six cases and a review. Br J Ophthalmol 69:681
Karakose S, Karabacakoglu A, Solak H (2002) Bilateral common carotid occlusion without neurological deficit. Australas Radiol 46:412–415
Kobayashi M, Kuroiwa T, Shimokawa R, Okeda R, Tokoro T (2000) Nitric oxide synthase expression in ischemic rat retinas. Jpn J Ophthalmol 44:235–244
Lee RM (1995) Morphology of cerebral arteries. Pharmacol Ther 66:149–173
Malhotra R, Gregory-Evans K (2000) Management of ocular ischaemic syndrome. Br J Ophthalmol 84:1428–1431
Miyamoto E, Nakao S, Tomimoto H, Wakita H, Yamada M, Masuzawa M, Takahira K, Sakamoto S, Shingu K (2004) Ketamine attenuates hypocapnia-induced neuronal damage in the caudoputamen in a rat model of chronic cerebral hypoperfusion. Neurosci Lett 354:26–29
Mizaner JB, Podhajsky P (1997) Ocular ischemic syndrome. Ophthalmology 104:859–864
Osborne NN, Block F, Sontag KH (1991) Reduction of ocular blood flow results in glial fibrillary acidic protein (GFAP) expression in rat retinal Muller cells. Vis Neurosci 7:637–639
Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J (2004) Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res 23:91–147
Osborne NN, Safa R, Nash MS (1999) Photoreceptors are preferentially affected in the rat retina following permanent occlusion of the carotid arteries. Vis Res 39:3995–4002
Otori T, Katsumata T, Katayama Y, Terashi A (1997) Measurement of regional cerebral blood flow and glucose utilization in rat brain under chronic hypoperfusion conditions following bilateral carotid artery occlusion. Analyzed by autoradiographical methods. Nippon Ika Daigaku Zasshi 64:428–439
Slakter JS, Spertus AD, Weissman SS, Henkind P (1984) An experimental model of carotid artery occlusive disease. Am J Ophthalmol 97:168–172
Stevens WD, Fortin T, Pappas BA (2002) Retinal and optic nerve degeneration after chronic carotid ligation: time course and role of light exposure. Stroke 33:1107–1112
Stys PK, Lesiuk H (1996) Correlation between electrophysiological effects of mexiletine and ischemic protection in central nervous system white matter. Neuroscience 71:27–36
Takamatsu J, Hirano A, Levy D, Henkind P (1984) Experimental bilateral carotid artery occlusion: a study of the optic nerve in the rat. Neuropathol Appl Neurobiol 10:423–428
Wakita H, Tomimoto H, Akiguchi I, Kimura J (1995) Protective effect of cyclosporin A on white matter changes in the rat brain after chronic cerebral hypoperfusion. Stroke 26:1415–1422
Wakita H, Tomimoto H, Akiguchi I, Lin JX, Ihara M, Ohtani R, Shibata M (2003) Ibudilast, a phosphodiesterase inhibitor, protects against white matter damage under chronic cerebral hypoperfusion in the rat. Brain Res 992:53–59
Wakita H, Tomimoto H, Akiguchi I, Matsuo A, Lin JX, Ihara M, McGeer PL (2002) Axonal damage and demyelination in the white matter after chronic cerebral hypoperfusion in the rat. Brain Res 924:63–70
Wilhelm H (1989) Tonic pupil caused by ischemia. Fortschr Ophthalmol 86:380–382
Acknowledgements
Financial support for this study was provided by PIBIC/CNPq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lavinsky, D., Sarmento Arterni, N., Achaval, M. et al. Chronic bilateral common carotid artery occlusion: a model for ocular ischemic syndrome in the rat. Graefe's Arch Clin Exp Ophthalmo 244, 199–204 (2006). https://doi.org/10.1007/s00417-005-0006-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-005-0006-7