Abstract
Background
Freezing of gait (FOG) is a common disabling gait disturbance among patients with Parkinson’s disease (PD), but the influence of genetic variants on the incidence of FOG has been poorly studied to date.
Objectives
We aimed to evaluate the association of GBA variants with the risk of FOG development in a large early PD cohort.
Methods
This study included 371 early PD patients from the Parkinson’s Progression Markers Initiative (PPMI) who were divided into a GBA variant carrier group (GBA-PD group, n = 44) and an idiopathic PD group without GBA variants (iPD group, n = 327). They were followed up for up to 5 years to examine the progression of FOG. The cumulative incidence of FOG and risk factors for FOG were assessed using Kaplan‒Meier and Cox regression analyses.
Results
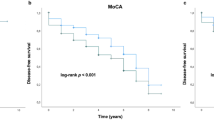
At baseline, the GBA-PD group had lower CSF β-amyloid 1–42 (Aβ42) levels and more severe motor and nonmotor symptoms than the iPD group. During the 5-year follow-up, the GBA-PD group had a higher incidence of FOG than the iPD group, and the FOG progression rate was related to GBA variant severity. In the multivariable Cox model without CSF Aβ42, GBA variants were significant predictors of future FOG, and the association remained significant after adding CSF Aβ42 to the model. In the subgroup analyses, the effect of GBA variants was not observed in the “low-level” group. However, in the “high-level” group, GBA variants independently increased the risk of FOG, and this association was stronger than the association with CSF Aβ42.
Conclusion
GBA variants are novel genetic risk factors for future FOG development in early PD patients. This association seemed to be mediated by both Aβ-dependent pathways and Aβ-independent pathways.



Similar content being viewed by others
Data availability
Data used in this study was downloaded from the Parkinsons Progression Markers Initiative (PPMI) database (https://adni.loni.usc.edu/) through a standard application process. For up-to-date information on the study, please visit https://adni.loni.usc.edu/.
References
Nutt J, Bloem B, Giladi N, Hallett M, Horak F, Nieuwboer A (2011) Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol 10:734–744
Perez-Lloret S, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destée A, Meissner W, Schelosky L, Tison F, Rascol O (2014) Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease. JAMA Neurol 71:884–890
Jung J, Kim B, Chung S, Yoo H, Lee Y, Baik K, Ye B, Sohn Y, Lee J, Lee P (2020) Motor cerebellar connectivity and future development of freezing of gait in de novo Parkinson’s disease. Move Disord 35:2240–2249
Nonnekes J, Snijders AH, Nutt JG, Deuschl G, Giladi N, Bloem BR (2015) Freezing of gait: a practical approach to management. Lancet Neurol 14:768–778
Kim R, Lee J, Kim HJ, Kim A, Jang M, Jeon B, Kang UJ (2019) CSF β-amyloid(42) and risk of freezing of gait in early Parkinson disease. Neurology 92:e40–e47
Gao C, Liu J, Tan Y, Chen S (2020) Freezing of gait in Parkinson’s disease: pathophysiology, risk factors and treatments. Transl Neurodegener 9:12
Weiss D, Schoellmann A, Fox M, Bohnen N, Factor S, Nieuwboer A, Hallett M, Lewis S (2020) Freezing of gait: understanding the complexity of an enigmatic phenomenon. Brain 143:14–30
Wang F, Pan Y, Zhang M, Hu K (2022) Predicting the onset of freezing of gait in Parkinson’s disease. BMC Neurol 22:213
Kim R, Lee J, Kim Y, Kim A, Jang M, Kim H, Jeon B, Kang U, Fahn S (2018) Presynaptic striatal dopaminergic depletion predicts the later development of freezing of gait in de novo Parkinson’s disease: an analysis of the PPMI cohort. Parkinsonism Relat Disord 51:49–54
Vercruysse S, Devos H, Munks L, Spildooren J, Vandenbossche J, Vandenberghe W, Nieuwboer A, Heremans E (2012) Explaining freezing of gait in Parkinson’s disease: motor and cognitive determinants. Mov Disord 27:1644–1651
Iansek R, Danoudis M (2017) Freezing of gait in Parkinson’s disease: its pathophysiology and pragmatic approaches to management. Mov Disord Clin Pract 4:290–297
Galvagnion C, Marlet F, Cerri S, Schapira A, Blandini F, Di Monte D (2022) Sphingolipid changes in Parkinson L444P GBA mutation fibroblasts promote α-synuclein aggregation. Brain 145:1038–1051
Vázquez-Vélez G, Zoghbi H (2021) Parkinson’s disease genetics and pathophysiology. Annu Rev Neurosci 44:87–108
Sidransky E, Nalls M, Aasly J, Aharon-Peretz J, Annesi G, Barbosa E, Bar-Shira A, Berg D, Bras J, Brice A, Chen C, Clark L, Condroyer C, De Marco E, Dürr A, Eblan M, Fahn S, Farrer M, Fung H, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang A, Lee-Chen G, Lesage S, Marder K, Mata I, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira L, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan E, Tayebi N, Toda T, Troiano A, Tsuji S, Wittstock M, Wolfsberg T, Wu Y, Zabetian C, Zhao Y, Ziegler S (2009) Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med 361:1651–1661
Neumann J, Bras J, Deas E, O’Sullivan S, Parkkinen L, Lachmann R, Li A, Holton J, Guerreiro R, Paudel R, Segarane B, Singleton A, Lees A, Hardy J, Houlden H, Revesz T, Wood N (2009) Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain 132:1783–1794
Zhang Y, Shu L, Zhou X, Pan H, Xu Q, Guo J, Tang B, Sun Q (2018) GBAA meta-analysis of -related clinical symptoms in Parkinson’s disease. Parkinson’s Dis 2018:3136415
Petrucci S, Ginevrino M, Trezzi I, Monfrini E, Ricciardi L, Albanese A, Avenali M, Barone P, Bentivoglio A, Bonifati V, Bove F, Bonanni L, Brusa L, Cereda C, Cossu G, Criscuolo C, Dati G, De Rosa A, Eleopra R, Fabbrini G, Fadda L, Garbellini M, Minafra B, Onofrj M, Pacchetti C, Palmieri I, Pellecchia M, Petracca M, Picillo M, Pisani A, Vallelunga A, Zangaglia R, Di Fonzo A, Morgante F, Valente E (2020) GBA-related Parkinson’s disease: dissection of genotype-phenotype correlates in a large Italian cohort. Mov Disord 35:2106–2111
Brockmann K, Srulijes K, Pflederer S, Hauser A, Schulte C, Maetzler W, Gasser T, Berg D (2015) GBA-associated Parkinson’s disease: reduced survival and more rapid progression in a prospective longitudinal study. Mov Disord 30:407–411
Alcalay R, Caccappolo E, Mejia-Santana H, Tang M, Rosado L, Orbe Reilly M, Ruiz D, Ross B, Verbitsky M, Kisselev S, Louis E, Comella C, Colcher A, Jennings D, Nance M, Bressman S, Scott W, Tanner C, Mickel S, Andrews H, Waters C, Fahn S, Cote L, Frucht S, Ford B, Rezak M, Novak K, Friedman J, Pfeiffer R, Marsh L, Hiner B, Siderowf A, Payami H, Molho E, Factor S, Ottman R, Clark L, Marder K (2012) Cognitive performance of GBA mutation carriers with early-onset PD: the CORE-PD study. Neurology 78:1434–1440
Malek N, Weil R, Bresner C, Lawton M, Grosset K, Tan M, Bajaj N, Barker R, Burn D, Foltynie T, Hardy J, Wood N, Ben-Shlomo Y, Williams N, Grosset D, Morris H (2018) GBAFeatures of -associated Parkinson’s disease at presentation in the UK study. J Neurol Neurosurg Psychiatry 89:702–709
Szwedo AA, Dalen I, Pedersen KF, Camacho M, Backstrom D, Forsgren L, Tzoulis C, Winder-Rhodes S, Hudson G, Liu G, Scherzer CR, Lawson RA, Yarnall AJ, Williams-Gray CH, Macleod AD, Counsell CE, Tysnes OB, Alves G, Maple-Grodem J, Parkinson’s Incidence Cohorts C (2022) GBA and APOE impact cognitive decline in Parkinson’s disease: a 10-year population-based study. Mov Disord 37:1016–1027
Morris R, Martini DN, Ramsey K, Kelly VE, Smulders K, Hiller A, Chung KA, Hu SC, Zabetian CP, Poston KL, Mata IF, Edwards KL, Lapidus J, Cholerton B, Montine TJ, Quinn JF, Horak F (2022) Cognition as a mediator for gait and balance impairments in GBA-related Parkinson’s disease. NPJ Parkinsons Dis 8:78
Choi S, Kim D, Kam TI, Yun S, Kim S, Park H, Hwang H, Pletnikova O, Troncoso JC, Dawson VL, Dawson TM, Ko HS (2015) Lysosomal enzyme glucocerebrosidase protects against abeta1-42 oligomer-induced neurotoxicity. PLoS ONE 10:e0143854
Brockmann K, Schulte C, Deuschle C, Hauser AK, Heger T, Gasser T, Maetzler W, Berg D (2015) Neurodegenerative CSF markers in genetic and sporadic PD: classification and prediction in a longitudinal study. Parkinsonism Relat Disord 21:1427–1434
Caminiti S, Carli G, Avenali M, Blandini F, Perani D (2021) Clinical and dopamine transporter imaging trajectories in a cohort of Parkinson’s disease patients with GBA mutations. Mov Disord 37:106–118
Nalls MA, Keller MF, Hernandez DG, Chen L, Stone DJ, Singleton AB (2016) Baseline genetic associations in the Parkinson’s Progression Markers Initiative (PPMI). Mov Disord 31:79–85
Davis M, Johnson C, Leverenz J, Weintraub D, Trojanowski J, Chen-Plotkin A, Van Deerlin V, Quinn J, Chung K, Peterson-Hiller A, Rosenthal L, Dawson T, Albert M, Goldman J, Stebbins G, Bernard B, Wszolek Z, Ross O, Dickson D, Eidelberg D, Mattis P, Niethammer M, Yearout D, Hu S, Cholerton B, Smith M, Mata I, Montine T, Edwards K, Zabetian C (2016) Association of GBA mutations and the E326K polymorphism with motor and cognitive progression in Parkinson disease. JAMA Neurol 73:1217–1224
Thaler A, Bregman N, Gurevich T, Shiner T, Dror Y, Zmira O, Gan-Or Z, Bar-Shira A, Gana-Weisz M, Orr-Urtreger A, Giladi N, Mirelman A (2018) Parkinson’s disease phenotype is influenced by the severity of the mutations in the GBA gene. Parkinsonism Relat Disord 55:45–49
Simuni T, Brumm M, Uribe L, Caspell-Garcia C, Coffey C, Siderowf A, Alcalay R, Trojanowski J, Shaw L, Seibyl J, Singleton A, Toga A, Galasko D, Foroud T, Nudelman K, Tosun-Turgut D, Poston K, Weintraub D, Mollenhauer B, Tanner C, Kieburtz K, Chahine L, Reimer A, Hutten S, Bressman S, Marek K (2020) Clinical and dopamine transporter imaging characteristics of leucine rich repeat kinase 2 (LRRK2) and glucosylceramidase beta (GBA) Parkinson’s disease participants in the Parkinson’s progression markers initiative: a cross-sectional study. Mov Disord 35:833–844
Wang C, Cai Y, Gu Z, Ma J, Zheng Z, Tang BS, Xu Y, Zhou Y, Feng T, Wang T, Chen SD, Chan P, Chinese Parkinson Study G (2014) Clinical profiles of Parkinson’s disease associated with common leucine-rich repeat kinase 2 and glucocerebrosidase genetic variants in Chinese individuals. Neurobiol Aging 35(725):e721-726
Mihaescu AS, Valli M, Uribe C, Diez-Cirarda M, Masellis M, Graff-Guerrero A, Strafella AP (2022) Beta amyloid deposition and cognitive decline in Parkinson’s disease: a study of the PPMI cohort. Mol Brain 15:79
Leocadi M, Canu E, Donzuso G, Stojkovic T, Basaia S, Kresojević N, Stankovic I, Sarasso E, Piramide N, Tomic A, Markovic V, Petrovic I, Stefanova E, Kostic V, Filippi M, Agosta F (2021) Longitudinal clinical, cognitive, and neuroanatomical changes over 5 years in GBA-positive Parkinson's disease patients. J Neurol
Kim R, Shin J, Park S, Kim H, Jeon B (2020) Apolipoprotein E ε4 genotype and risk of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord 81:173–178
Sardi S, Cheng S, Shihabuddin L (2015) Gaucher-related synucleinopathies: the examination of sporadic neurodegeneration from a rare (disease) angle. Prog Neurobiol 125:47–62
Blandini F, Cilia R, Cerri S, Pezzoli G, Schapira A, Mullin S, Lanciego J (2019) Glucocerebrosidase mutations and synucleinopathies: toward a model of precision medicine. Mov Disord 34:9–21
Lerche S, Schulte C, Wurster I, Machetanz G, Roeben B, Zimmermann M, Deuschle C, Hauser A, Böhringer J, Krägeloh-Mann I, Waniek K, Lachmann I, Petterson X, Chiang R, Park H, Wang B, Liepelt-Scarfone I, Maetzler W, Galasko D, Scherzer C, Gasser T, Mielke M, Hutten S, Mollenhauer B, Sardi S, Berg D, Brockmann K (2021) The mutation matters: CSF profiles of GCase, sphingolipids, α-synuclein in PD. Mov Disord 36:1216–1228
Avenali M, Blandini F, Cerri S (2020) Glucocerebrosidase defects as a major risk factor for Parkinson’s disease. Front Aging Neurosci 12:97
Behl T, Kaur G, Fratila O, Buhas C, Judea-Pusta C, Negrut N, Bustea C, Bungau S (2021) Cross-talks among GBA mutations, glucocerebrosidase, and α-synuclein in GBA-associated Parkinson’s disease and their targeted therapeutic approaches: a comprehensive review. Transl Neurodegener 10:4
Acknowledgements
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org. PPMI (a public–private partnership) was funded by the Michael J. Fox Foundation for Parkinson’s Research and multiple funding partners, including AbbVie, Avid, Biogen, Bristol-Myers Squibb, Covance, GE Healthcare, Genentech, GlaxoSmithKline, Lilly, Lundbeck, Merck, Meso Scale Discovery, Pfizer, Piramal, Roche, Servier, Teva, and UCB. The authors should thank the Department of Translational Medicine Center of the First Affiliated Hospital of Zhengzhou University for their technical support.
Funding
This study was supported by grants from the China Postdoctoral Science Foundation funded project (No. 2021M702945), the Henan Medical Science and Technology Joint Building Program (No. LHGJ20210300) and the starting fund for postdoctoral research of the First Affiliated Hospital of Zhengzhou University to Nannan Yang, the National Natural Science Foundation of China (No. 82101940 to Shushan Sang and No. 81971175 to Hong Lu).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical standard statement
The data were retrieved from the PPMI, an online database that provides de-identifed clinical data. That study was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice (GCP) guidelines after approval of the local ethics committees of the participating sites. For up-to-date information on the study, visit https://adni.loni.usc.edu/.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, N., Sang, S., Peng, T. et al. Impact of GBA variants on longitudinal freezing of gait progression in early Parkinson’s disease. J Neurol 270, 2756–2764 (2023). https://doi.org/10.1007/s00415-023-11612-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11612-6