Abstract
Patients with post-coronavirus disease 2019 (COVID-19) conditions typically experience cognitive problems. Some studies have linked COVID-19 severity with long-term cognitive damage, while others did not observe such associations. This discrepancy can be attributed to methodological and sample variations. We aimed to clarify the relationship between COVID-19 severity and long-term cognitive outcomes and determine whether the initial symptomatology can predict long-term cognitive problems. Cognitive evaluations were performed on 109 healthy controls and 319 post-COVID individuals categorized into three groups according to the WHO clinical progression scale: severe-critical (n = 77), moderate-hospitalized (n = 73), and outpatients (n = 169). Principal component analysis was used to identify factors associated with symptoms in the acute-phase and cognitive domains. Analyses of variance and regression linear models were used to study intergroup differences and the relationship between initial symptomatology and long-term cognitive problems. The severe-critical group performed significantly worse than the control group in general cognition (Montreal Cognitive Assessment), executive function (Digit symbol, Trail Making Test B, phonetic fluency), and social cognition (Reading the Mind in the Eyes test). Five components of symptoms emerged from the principal component analysis: the “Neurologic/Pain/Dermatologic” “Digestive/Headache”, “Respiratory/Fever/Fatigue/Psychiatric” and “Smell/ Taste” components were predictors of Montreal Cognitive Assessment scores; the “Neurologic/Pain/Dermatologic” component predicted attention and working memory; the “Neurologic/Pain/Dermatologic” and “Respiratory/Fever/Fatigue/Psychiatric” components predicted verbal memory, and the “Respiratory/Fever/Fatigue/Psychiatric,” “Neurologic/Pain/Dermatologic,” and “Digestive/Headache” components predicted executive function. Patients with severe COVID-19 exhibited persistent deficits in executive function. Several initial symptoms were predictors of long-term sequelae, indicating the role of systemic inflammation and neuroinflammation in the acute-phase symptoms of COVID-19.” Study Registration: www.ClinicalTrials.gov, identifier NCT05307549 and NCT05307575.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The post-coronavirus disease 2019 (COVID-19) condition (PCC) manifests 3 months after the onset of the disease, and presents with symptoms that remain for at least 2 months and cannot be explained by other diseases [1]. PCC is characterized by a wide variety of fixed or fluctuating symptoms, including cognitive complaints. While 60%-80% of patients with PCC report experiencing brain fog, memory, loss of attentional focus, and language disturbances [2,3,4], objective evaluations of people with PCC have shown impairments in attention, processing speed, memory, and executive functions [5,6,7].
The severity of COVID-19 and post-COVID cognitive impairment assessed through systematic neuropsychological assessments was first shown to be related in hospitalized patients with acute disease [8]. Intensive care unit (ICU) stay has been linked to reduced executive function, and the need for oxygen therapy has been linked to reduced performance in several cognitive measures 10–40 days after hospital discharge. Over the medium-long term, the general severity of acute illness has been related to residual cognitive deficits [9], treatment required for respiratory symptoms has been related to worse global cognitive performance [10], respiratory distress to lower processing speed [11], and hypoxemia to impaired long-term memory and visuospatial learning at five months but not at the one-year evolution [12].
Additional evidence has been obtained from studies comparing hospitalized and non-hospitalized patients. In comparison with non-hospitalized patients, hospitalized individuals are more likely to have impairments in attention, executive functioning, category fluency, and verbal memory [13] or slower processing speed [5]. Post-ICU patients showed a lower cognitive composite score than non-ICU patients. However, among non-ICU patients, the cognitive composite score did not differ between those who were hospitalized and those who were not [14]. In a similar study performed with a healthy control (HC) group, patients with severe PCC showed lower processing speed than those with mild-moderate PCC and healthy control participants [15]. In a Finnish study, both ICU and hospitalized patients underperformed patients treated at home in the total cognitive score at 6 months post-COVID. Moreover, ICU participants underperformed hospitalized patients and HCs in the attention domain [16].
However, in multiple investigations using samples from 18 to 478 hospitalized and non-hospitalized participants with acute illnesses, the severity of COVID-19 was not associated with cognitive impairments at 3–4 months [17,18,19]. According to a recent meta-analysis, patients admitted to the hospital during the acute infection were less likely to report post-COVID cognitive symptoms than outpatients three months (or more) after the disease [20].
Another aspect that requires consideration is the predictive value of acute symptomatology for long-term cognitive impairment. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can infect several human cell types, as seen by COVID-19's vast array of symptoms. Typical signs and symptoms include fever, fatigue, gastrointestinal issues, cough, sore throat, shortness of breath, myalgia, headaches, dizziness, and changes in smell and taste [21,22,23]. The etiology of cognitive dysfunction may originate from the pathophysiology of acute illness [24]. However, it is currently unknown whether the effects of COVID-19 on the brain are caused by virus invasion in the brain, oxygen deprivation of the brain, or the body's excessive inflammatory response in seriously affected individuals [25]. Acute symptoms, even if they are not neurological manifestations, could contribute to the understanding of post-COVID cognitive problems. In a split study, Guo et al. found that initial illness-related symptoms explained part of the variation in post-COVID subjective cognitive symptoms [3]. They then demonstrated how some aspects of neuropsychological performance can also be explained by acute sickness symptoms [26].
Despite the number of studies in the field, the relationship between cognitive outcomes and the severity of COVID-19 is still not completely clear, probably because the underlying mechanisms of the cognitive deficits identified are mostly unknown. This study aimed to clarify the relationship between the severity of COVID-19 and long-term cognitive outcomes in a large sample of participants, including a control group. Our second objective was to determine if the initial symptomatology can predict long-term cognitive impairment. Since COVID-19 symptoms are highly diverse and heterogeneous, we aimed to use principal component analysis to identify phenotypes or clinical symptoms that frequently coexist.
Methods
Participants
The NAUTILUS Project is a cross-sectional observational study of post-COVID-19 cognitive consequences based on multimodal data. We used the available clinical and neuropsychological data of the project in the present study. The sample consisted of 428 participants, including 319 participants with PCC and 109 HC individuals who were evaluated at the Neuropsychology and COVID Units of 16 Hospitals in Catalonia, Madrid, and Andorra, coordinated by the Consorci Sanitari de Terrassa (Terrassa, Barcelona, Spain). The inclusion criteria for the PCC group were as follows: (a) confirmed diagnosis of COVID-19 according to WHO criteria with signs and symptoms of the disease during the acute phase; (b) at least 12 weeks after infection; and (c) age over 18 years. Exclusion criteria were as follows: (a) established diagnosis of psychiatric, neurological, neurodevelopmental disorder, or systemic pathologies known to cause cognitive deficits before the episode of COVID-19, and (b) motor or sensory alterations that impeded neuropsychological examination. The HCs did not have COVID-19 (no positive test or compatible symptoms) and were selected after applying the same exclusion criteria as in the PCC group. All participants were native Spanish speakers.
Procedure
The overall procedure consisted of two sessions. In the first session, various questionnaires were administered to collect information about demographic factors and behaviors related to the participants’ health and medical history. Participants with PCC were questioned about their COVID-19 experience and the symptoms they were experiencing at the time of evaluation. For a list of typical acute COVID-19 acute symptoms, presence/absence and the number of days were recorded. We developed a scale from 0 to 4, in which 0 indicated the absence of the symptom and 4 indicated a long-lasting symptom. Next, participants rated the severity of their COVID-19 experience on a visual analog scale of 1–10. Later, they were asked about the symptoms they were currently experiencing (post-COVID symptoms) and whether these were minor, major, or different from those experienced in the acute phase. Finally, we asked them to report any other symptoms they had been experiencing and had not been covered in the interview.
In the second session, each participant underwent a cognitive assessment with a comprehensive neuropsychological battery. We used the Montreal Cognitive Assessment (MoCA) for general cognitive screening [27, 28]. The WAIS-IV Digit Span subtest was used to measure verbal attention (digit span forward) and working memory (digit span backward) [29]. To assess verbal memory, we used the Spanish version of Rey's Auditory Verbal Learning Test (RAVLT) [30, 31]. Visual scanning, tracking, and motor speed were assessed by the Digit Symbol Coding Test (WAIS-III) [29]. Parts A and B of the Trail Making Test (TMT) were administered to measure visual scanning, motor speed and attention, and mental flexibility [32]. A difference score (B-A) that removed the speed element from the test evaluation was calculated [33]. The Controlled Oral Word Association Test (COWAT) [34, 35] was used to evaluate verbal fluency and language. The number of words beginning with the letters P, M, and R recalled in 1 min was recorded. Semantic fluency was evaluated using the category “animals” [36]. The number of correct animals reported in 1 min was counted. The interference score of the Stroop test was calculated as a measure of cognitive inhibitory control [37]. The Boston Naming Test (BNT) was used to evaluate language [38]. Social cognition was assessed with the Reading the Mind in the Eye Test (RMET) [39]. The Word Accentuation Test (TAP) was used to estimate the premorbid intelligence quotient (IQ) [40]. In addition to cognitive measures, we used the Chalder Fatigue Scale (CFQ) [41] to assess fatigue, the Generalized Anxiety Disorder 7-item scale (GAD-7) [42, 43] to assess anxiety, and the Patient Health Questionnaire-9 (PHQ-9) to assess depressive symptoms [44, 45]. The quality of life was evaluated by the WHOQOL-BREFF [46]. Trained neuropsychologists performed all evaluations.
The recruitment was conducted between June 2021 and June 2022. The study was conducted with the approval of the Drug Research Ethics Committee (CEIm) of Consorci Sanitari de Terrassa (CEIm code: 02–20-107–070) and the Ethics Committee of the University of Barcelona (IRB00003099). All participants provided written informed consent.
Statistical analyses
Descriptive statistics were obtained for all variables of the study. Group differences in demographics were examined by conducting an analysis of variance (ANOVA). The Chi-square test was performed to compare binarized measures between the groups. One-way analysis of covariance (ANCOVA) with Bonferroni-adjusted post-hoc comparisons was performed to determine group differences in cognitive functioning. Graphical representations and descriptive statistics were used to study the assumptions. The effect size was calculated using the value partial eta squared (ήp2). To investigate if the cognitive symptoms of PCC were predicted by the acute-phase symptoms, principal component analysis (PCA) was performed first on 21 auto-reported acute-phase symptoms and on Z-scores of 15 neuropsychological variables to define the cognitive domains, followed by linear regressions (stepwise) with the acute symptom components as predictors and the neuropsychological components as dependent variables. Analyses were performed using IBM SPSS Statistics 27.0 (IBM Corporation, Armonk, NY, USA) and R Statistical Software (version 4.2.0; The R Foundation for Statistical Computing Platform). The critical level for statistical significance was set at α = 0.05.
Results
Sample demographics
The 319 participants with PCC were classified into three groups according to the WHO clinical progression scale [47]: severe-intensive care unit (ICU-PCC) (n = 77), hospitalized (H-PCC) (n = 73), and mild (M-PCC) (n = 169) (Table 1). The participants’ sociodemographic characteristics and comorbidities are shown in Table 2. The M-PCC and the HC groups were equivalent in age and sex, had a higher proportion of women, and were younger than the ICU-PCC and the H-PCC groups. The three PCC groups showed no differences in formal education and estimated IQ. However, the education level and estimated IQ in the HC group were higher than those in all three PCC groups. Thus, age, sex, educational level, and estimated IQ were covariates in comparing cognitive results among the four groups. On average, all PCC participants had shown a positive test 320 days before their neuropsychological evaluation (SD = 156.66 days), and the ICU-PCC group had fewer days of evolution since the start of COVID-19 than the other two groups. Premorbid high blood pressure and obesity were more prevalent among ICU participants than the other PCC and HC groups.
Differences in cognitive performance
Table 3 shows the fatigue, depression, anxiety, and quality of life scores for each PCC severity and HC group. The CFQ, PHQ-9, GAD-7, and WHOQOL-BREF scores were significantly different among groups. Post-hoc analysis showed that the CFQ, PHQ-9, and GAD-7 scores were higher in the PCC than in the HC group. Individuals in the M-PCC group had higher fatigue and depression levels than those in the H-PCC group. The quality of life assessed by the WHOQOL-BREFF was better in the HC group than in the PCC groups. We used fatigue, depression, and anxiety as covariates in the cognitive analysis. However, we also analyzed the data without these mood and fatigue variables (Supplementary Table 1).
The groups showed statistically significant differences in MoCA, Digit symbol, TMT-B, TMT-B-A, phonetic fluency, and the RMET scores after controlling for age, sex, educational level, estimated IQ, fatigue, depression, and anxiety test scores. The ICU-PCC group performed worse in the MoCA, Digit symbol, TMT B, TMT-B-A, phonetic fluency, and RMET assessments than the HC group and obtained poorer results than the M-PCC group in the TMT-B and TMT-B-A assessments. The H-PCC group showed worse performance in the Digit symbol assessments than the HC group (Table 4 and Fig. 1).
Cognitive profiles of the post-COVID condition severity groups and healthy controls. Healthy controls (HCs) are presented in green, ICU-PCC in blue, H-PCC in yellow, and M-PCC in red. Data are presented as means of Z-scores adjusted by age, sex, educational level, estimated IQ, fatigue, depression, and anxiety test scores. Lower Z-scores indicate poorer performance, except for TMT (time), where lower Z-scores indicate better performance. Statistically significant differences between groups are marked with an asterisk
Table 5 shows the frequency of acute-phase symptoms for each severity group and all the PCC participants. ICU stay was associated with greater limb weakness and the presentation of delirium and psychotic symptoms. Hospitalization was associated with fever. A higher proportion of PCC participants at home had headache, muscle and joint pain, changes in smell and taste, nasal congestion, and sore throat. The three groups did not show differences in the perception of COVID-19 severity measured with the visual analog scale (ICU: mean = 7.91, SD = 2.22; H: mean = 7.86, SD = 1.65; M: mean = 7.05, SD = 2.41).
Effect of acute symptoms on long-term cognition
PCA with initial symptoms was performed with a varimax orthogonal rotation to facilitate interpretability. The Kaiser–Meyer–Olkin (KMO) value (0.834) and Bartlett's test of sphericity (χ2(210) = 1571.92; p < 0.000) indicated that the data were likely factorizable. PCA revealed five components with eigenvalues more significant than one, which explained 24.92%, 8.17%, 6.56%, 5.71%, and 5.11% of the total variance and were classified as “Digestive/Headache” (nausea, loss of appetite, dizziness, diarrhea, shaking chills, and headache), “Respiratory/Fever/Fatigue/Psychiatric” (depressive symptoms, anxious symptoms, psychotic symptoms, breathing issues, fever, and fatigue), “Neurologic/Pain/Dermatologic” (paresthesia, skin problems, limb weakness, and muscle and joint pain), “Smell/Taste” (smell and taste symptoms), and “Cold” (nasal and conjunctival congestion and cough), respectively. The factor scores were computed through the regression method. The rotated (varimax) component loadings for the initial symptoms are shown in Table 6.
The scores for the Digestive/Headache, Respiratory/Fever/Fatigue/Psychiatric, and Smell/Taste components were significantly different among the severity groups. Post-hoc analysis showed that the Digestive/Headache score was higher in the M-PCC group than in the ICU-PCC group; the Respiratory/Fever/Fatigue/Psychiatric score was higher in the ICU-PCC and H-PCC groups than in the M-PCC group, and the Smell/Taste score was higher in the M-PCC than in the ICU-PCC and H-PCC groups (Fig. 2 and Supplementary Table 2).
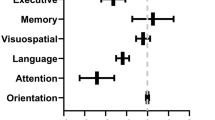
PCA with neuropsychological variables was performed with a direct oblimin rotation to facilitate interpretability. We excluded the scores obtained with the MoCA (a screening tool covering several cognitive domains) and the RMET (social cognition domain). All assumptions were met: overall KMO = 0.910 and Bartlett's test (χ2(105) = 3878.99, p = 0.0001). PCA revealed four components as the best factorial solution, which explained 72.71% of the total variance (45.14%, 12.86%, 8.14%, and 6.57%). We classified the four components as the following cognitive domains: executive function (TMT, Symbol Digit, Stroop task), verbal memory (RAVLT), attention and working memory (WM) (Digits span forward and backward), and language (Phonetic fluency, Semantic fluency, BNT). The regression approach was used to calculate the factor scores. Component loadings of the rotated solution are presented in Table 7. Figure 3 shows the profile of the cognitive domains for the PCC severity and HC groups corrected for age, sex, educational level, time of evolution, fatigue, and depression test scores.
Cognitive domain profiles for the post-COVID conditions severity groups. ICU-PCC in blue, H-PCC in yellow, and M-PCC in red. Data are presented as means of Z-scores (adjusted by age, sex, educational level, time of evolution, fatigue, and depression test scores) and deviation error bars. Lower Z-scores indicate poorer performance. Statistically significant differences were noted between PCC severity groups (marked with an asterisk)
Linear regressions (stepwise) with the five acute symptom components as predictors and the neuropsychological components as dependent variables were performed. In addition to the four cognitive components, MoCA and RMET scores were used as dependent variables in multiple linear regression. The linear regression models were adjusted for potential confounders (age, sex, years of education, time of evolution, premorbid high blood pressure and obesity).
As seen in Table 8, the “Neurologic/Pain/Dermatologic”, “Digestive/Headache”, “Respiratory/Fever/Fatigue/Psychiatric” and “Smell/ Taste” components added statistical significance to the prediction of MoCA scores. Executive function was predicted by the “Respiratory/Fever/Fatigue/Psychiatric,” “Neurologic/Pain/Dermatologic,” and “Digestive/Headache” components. The “Neurologic/Pain/Dermatologic” and “Respiratory/Fever/Fatigue/Psychiatric” components added statistical significance to the prediction of verbal memory scores, and the attention and WM component was predicted by the “Neurologic/Pain/Dermatologic” component. The language and social cognition components were not explained by any acute-phase symptom component but by the variables for demographic characteristics and premorbid conditions.
Discussion
The primary objective of the present study was to elucidate the link between COVID-19 severity and long-term cognitive outcomes. Previous studies have shown inconsistent results: some have reported a relationship [5, 8,9,10,11,12,13,14,15,16], while others did not identify any severity variable explaining cognitive performance [17,18,19,20]. Comparisons of these studies are challenging because their conclusions were drawn using various designs and methodologies. Moreover, only a few studies were specifically designed to examine this association [16, 18]. Some studies did not categorize patients according to the severity of their acute illness [8, 9, 11, 12, 19], or if they did, this categorization was only partially done or did not include a control group [5, 10, 13,14,15, 18]. Other studies only correlated the results of selected cognitive tests with severity assessments [10,11,12, 17]. Only one previous study compared groups according to the acute care environment and employed an HC group [16].
The neuropsychological performance profile obtained in our study with 428 participants showed a gradation in the expected direction: ICU-PCC < H-PCC < M-PCC < HC. After controlling for the variables that differed between groups, we found significant differences for the six neuropsychological tests. Post-hoc group comparisons showed that the significant differences arose mainly from the contrast between the HC and ICU-PCC groups. These tests measured global cognition (MoCA), executive functions-mental processing speed (Digit symbol, TMT-B, Phonetic Fluency), and social cognition (RMET). Additionally, the TMT-B test distinguished between ICU-PCC and M-PCC participants.
Our findings partially agreed with those of a study with 213 participants and a similar design to ours [16]. In that study, the severity of COVID-19 was related to deterioration in an overall cognitive score and the attention domain. Some of the tests used to define the attentional domain in that study were also used in our study (Digit symbol, Stroop), while one test that was not used in the present study (Continuous Performance Test) was more sensitive to attention. Although depression and post-traumatic stress disorder were controlled in their overall score analysis, they were not controlled in the attention analysis. The authors of that study reported a relationship between executive function impairment and severity, but this relationship was observed only in men. In our sample, this relationship appeared regardless of sex. Our results referring to the relationship between executive function impairment and the severity of COVID-19 also agree with those of another study [13]. However, that study did not distinguish between hospitalized and ICU participants. The hospitalized patients in our sample did not differ from the outpatients in any test. In contrast, the ICU patients differed from the outpatients in two measures.
Although the neuropsychological profile indicates impairment in the executive domain, tests grouped under executive functions can also be considered to involve processing speed. Several previous studies have related slowness with illness severity [5, 11, 15]. Our results support this relationship. Long-term slower mental speed processing has been linked to hypoxemia in individuals with acute respiratory distress syndrome (ARDS) [48]. Silent hypoxemia is a common feature in SARS-CoV-2 infections [49]. This trait caused delays in patient treatment, particularly during the first wave of the pandemic, which worsened the patients’ prognosis [50]. The integrity of white matter across the brain is related to processing speed and, more generally, to intellectual ability [51, 52]. White matter intensities have been shown to be associated with nocturnal hypoxemia [53] and hypoxic-ischemic brain injury in COVID-19-related ARDS [54]. Consistent with these findings, effects on the white matter have been reported to occur a year after COVID-19, specifically in the corona radiata, corpus callosum, and superior longitudinal fasciculus, particularly in post-ICU individuals [55]. COVID-19-induced white matter injury may be mediated by hypoxia as well as indirect viral invasion [56, 57], the systemic inflammatory response [58], or coagulopathy [59].
COVID-19 severity was not related to memory in our research, even though this relationship has been reported previously [12,13,14]. This result was unexpected due to the poor memory performance in the entire sample of PCC individuals in comparison with the HC group in our previous study [7]. The high prevalence of depression and anxiety symptoms and fatigue in our groups may explain this finding. In our previous study, fatigue, depression, and anxiety symptoms explained part of the memory performance variance in our PCC groups. Here, when we analyzed the data without controlling for emotional variables and fatigue, the H-PCC and M-PCC participants' learning was inferior to that in the HC group. In addition, the M-PCC group demonstrated poorer long-term memory and recognition than the HC group. Numerous studies have found a link between depression and memory problems in post-COVID individuals [60,61,62]. The causal connection between depression and memory impairment is, however, uncertain.
In contrast to the findings reported in other studies [10, 63], we did not find differences in cognitive impairment between M-PCC and HC participants. The previous studies performed cognitive assessments of participants 3–6 months after the positive COVID-19 test. In contrast, cognitive assessments for the M-PCC group in the present study were performed an average of eleven months from the acute infection, when most participants may have recovered, at least in part. Most post-COVID symptoms decrease between 3 and 12 months [64], and this change has also been reported in the cognitive symptoms [12]. One study showed no differences between patients with mild- moderate COVID-19 and HCs 4 months post-infection. However, the groups in that study showed remarkable differences in anxiety, depression, and stress [62]. On the other hand, one study evaluating mild COVID-19 individuals at 11 months found several impaired cognitive measures relative to HC. Nevertheless, these authors did not assess whether their participants had fatigue or mood disturbances [65].
Different pathophysiological pathways for brain damage are probably implicated in mild, hospitalized, and critical cases of COVID-19. Despite the possibility of shared pathophysiological mechanisms, assumptions can be made for each group of patients. Mild cases may be caused directly by the virus (olfactory channel of entry) [66, 67]. The degree of systemic inflammation and level of hypoxemia are presumably higher in moderate-COVID-19 individuals [68]. In addition to more severe hypoxemia, systemic inflammation, and organ failure, brain injury may result from ICU therapies, including bed rest, life support equipment, and drugs in critical patients [69].
As a second aim, we investigated the relationship between acute symptoms and long-term cognitive outcomes. We identified five acute symptom components and found correlations between some of these components and long-term cognitive performance. “Neurologic/Pain/Dermatologic,” “Digestive/Headache, Respiratory/Fever/Fatigue/Psychiatric,” and “Smell/Taste” predicted 28% of the variance in global cognition. The “Neurologic/Pain/Dermatologic” component also explained 12% of the variance in attention and WM, and the “Neurologic/Pain/Dermatologic” and “Respiratory/Fever/Fatigue/Psychiatric” components together explained 23% of variance in verbal memory. Finally, 24% of the variance in executive function was accounted for the “Neurologic/Pain/Dermatologic,” “Respiratory/Fever/Fatigue/Psychiatric,” and “Digestive/Headache” components. These three components included the symptoms limb weakness, paresthesia, muscle and joint pain, respiratory issues, fever, depression, anxiety, psychotic symptoms, fatigue, dizziness, and headache.
These results may provide insights into the mechanisms underlying cognitive changes. The fact that the initial symptoms explain some of the variations in long-term cognition suggests that the brain regions responsible for these cognitive tasks were affected, and some of this impairment may have occurred during the acute phase of the illness. The Neurological/Pain/Dermatological, Respiratory/Fever/Fatigue/Psychiatric and Digestive/Headache components included symptoms that develop during systemic inflammation (pain, fatigue, fever, limb weakness, and paresthesia) and neuroinflammation (headache, dizziness, limb weakness, paresthesia, and mood alterations), although these components cannot be explained in terms of inflammation.
We speculate that long-term cognitive impairment could have been caused by sustained systemic or neurological inflammation. Infections result in systemic inflammation and are associated with activation of microglial cells and the appearance of cognitive deficits. Neuroinflammation is caused by activation of microglial cells and the overexpression of proinflammatory cytokines, both of which are induced by the peripheral immune system [70].
In the initial phase of the study, critical patients showed impairment in global cognition, executive function, and social cognition. The variance of these cognitive areas is partially explained here by acute symptom variables. In addition to the inflammation mechanisms underlying the Neurologic/Pain/Dermatologic and Respiratory/Fever/Fatigue/Psychiatric factors, the added Digestive/Headache factor provides an alternative pathophysiological mechanism to explain executive function impairment. The hypothalamus regulates symptoms such as nausea and loss of appetite. SARS-CoV-2 has been suggested to use the nervus terminalis rather than the olfactory nerve as a direct pathway to infect the brain from the nasal cavity [71]. Bypassing the olfactory bulb, nerve terminal neurons project straight to locations in the brain, including the hypothalamus. Infection of the hypothalamus can produce these symptoms and allow the infection to spread to the medial prefrontal lobe [72], contributing to the pathophysiology of executive dysfunction.
Although the results of the verbal memory, attention, and working memory tests did not differ significantly between groups in the initial phase of the study, models predicting the early symptomatology were identified for the components corresponding to these tests. However, language impairments were not predicted by any symptom factor. Instead, these impairments were predicted by demographic variables. Since emotion recognition is associated with the orbitofrontal cortex and temporal regions, we anticipated that the route of entry of the virus through the olfactory system could cause damage to these structures. However, patients with milder disease were more likely to experience impairments in smell and taste, whereas the ICU group, with the most severe condition, demonstrated the poorest social cognition. In this model, obesity, a chronic inflammatory condition [73] linked to the severity of COVID-19 [74], served as an explanatory variable. In addition to the risk posed by chronic inflammation, severely obese patients show considerable management issues in the ICU, particularly for the respiratory level [75]. Therefore, the impairment of the brain structures responsible for recognizing emotions should be attributed to indirect mechanisms, such as hematogenous pathways of virus entry to the central nervous system or systemic inflammatory mechanisms, and not to the direct action of the virus. We cannot rule out the possibility that sedated and intubated participants’ self-reported baseline symptoms were not as accurate as those with less severe COVID-19. Thus, we may have lacked complete and reliable data regarding symptoms such as anosmia/ageusia in severely ill patients.
The limitations and strengths of the study require consideration while interpreting the findings. A major limitation refers to the collection of initial symptoms, which were self-reported through a questionnaire in the first session with the patient; the questionnaire itself was based on the symptoms most frequently reported in the literature. Thus, the presence of initial symptoms was recorded and scored retrospectively, which may have introduced recall bias. Moreover, we collected data for the presence and duration but not the intensity of each symptom. We did not use objective severity measures such as hypoxemia, days of sedation or weaning, or blood inflammatory levels, and the analysis was based solely on the reported symptoms. Since these factors may better explain the cognitive deficit, these variables will be examined in depth in future studies to understand the pathogenesis of cognitive dysfunction in PCC individuals.
On the other hand, our sample size was reasonably large and represented the full spectrum of COVID-19 severity. Although the control group was not optimal because we had to control for some variables statistically, it was tested simultaneously with the COVID-19 participants, with the HCs experiencing the same pandemic circumstances. Unlike other studies, our participants were selected on the basis of inclusion criteria that precluded the presence of neurological, psychiatric, or systemic illnesses before COVID-19, conditions that could have influenced the cognitive findings. In addition, the cognitive examination was carried out in person with an extensive neuropsychological battery commonly used in the clinical context, which validated its applicability.
In conclusion, the results of this study showed evident long-term impairments in patients with severe COVID-19 requiring ICU admission, although hospitalization per se did not involve long-term neuropsychological sequelae. Global cognition, executive function, and social cognition were the domains most affected by the severity of COVID-19. For the initial symptomatology, the factors Neurologic/Pain/Dermatologic, Respiratory/Fever/Fatigue/Psychiatric, and Digestive/Headache explained part of the variance of global cognition, attention and working memory, verbal memory and executive function.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Soriano JB, Murthy S, Marshall JC et al (2022) A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis 22:e102–e107. https://doi.org/10.1016/S1473-3099(21)00703-9/ATTACHMENT/EF4FD06B-88FA-4A0C-B837-DCFEE13E82D7/MMC1.PDF
Davis HE, Assaf GS, McCorkell L, et al (2021) Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 38:101019. https://doi.org/10.1016/J.ECLINM.2021.101019/ATTACHMENT/499C606A-AE36-49F5-87DD-09E3B87369C9/MMC1.DOCX
Guo P, Benito Ballesteros A, Yeung SP, et al (2022) COVCOG 1: factors predicting physical, neurological and cognitive symptoms in long COVID in a community sample. a first publication from the COVID and cognition study. Front Aging Neurosci 14:. https://doi.org/10.3389/FNAGI.2022.804922/FULL
Ziauddeen N, Gurdasani D, O’Hara ME, et al (2022) Characteristics and impact of Long Covid: Findings from an online survey. PLoS One 17:e0264331. https://doi.org/10.1371/JOURNAL.PONE.0264331
García‐Sánchez C, Calabria M, Grunden N, et al (2022) Neuropsychological deficits in patients with cognitive complaints after COVID‐19. Brain Behav e2508. https://doi.org/10.1002/brb3.2508
Delgado-Alonso C, Valles-Salgado M, Delgado-Álvarez A et al (2022) Cognitive dysfunction associated with COVID-19: A comprehensive neuropsychological study. J Psychiatr Res 150:40–46. https://doi.org/10.1016/j.jpsychires.2022.03.033
Ariza M, Cano N, Segura B, et al (2022) Neuropsychological impairment in post-COVID condition individuals with and without cognitive complaints. Front Aging Neurosci 1–12. https://doi.org/10.3389/fnagi.2022.1029842
Almeria M, Cejudo JC, Sotoca J, et al (2020) Cognitive profile following COVID-19 infection: Clinical predictors leading to neuropsychological impairment. Brain Behav Immun Health 9:100163. https://doi.org/10.1016/J.BBIH.2020.100163
Hampshire A, Chat A, Mphil M, et al (2022) Multivariate profile and acute-phase correlates of cognitive deficits in a COVID-19 hospitalised cohort. EClinical Medicine 47:1–10. https://doi.org/10.1016/j.eclinm.2022.101417
Hampshire A, Trender W, Chamberlain SR, et al (2021) Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine 39:101044. https://doi.org/10.1016/j.eclinm.2021.101044
Cecchetti G, Agosta F, Canu E et al (2022) Cognitive, EEG, and MRI features of COVID-19 survivors: a 10-month study. J Neurol 269:3400–3412. https://doi.org/10.1007/s00415-022-11047-5
Ferrucci R, Dini M, Rosci C et al (2022) One-year cognitive follow-up of COVID-19 hospitalized patients. Eur J Neurol 29:2006. https://doi.org/10.1111/ENE.15324
Becker JH, Lin JJ, Doernberg M et al (2021) Assessment of cognitive function in patients after COVID-19 infection. JAMA Netw Open 4:e2130645–e2130645. https://doi.org/10.1001/JAMANETWORKOPEN.2021.30645
Vannorsdall TD, Brigham E, Fawzy A et al (2022) Cognitive dysfunction, psychiatric distress, and functional decline after COVID-19. J Acad Consult Liaison Psychiatry 63:133–143. https://doi.org/10.1016/J.JACLP.2021.10.006
Santoyo-Mora M, Villaseñor-Mora C, Cardona-Torres LM et al (2022) COVID-19 Long-term effects: is there an impact on the simple reaction time and alternative-forced choice on recovered patients? Brain Sci 12:1258. https://doi.org/10.3390/BRAINSCI12091258
Ollila H, Pihlaja R, Koskinen S et al (2022) Long-term cognitive functioning is impaired in ICU-treated COVID-19 patients: a comprehensive controlled neuropsychological study. Crit Care 26:1–11. https://doi.org/10.1186/s13054-022-04092-z
Woo MS, Malsy J, Pöttgen J et al (2020) Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. https://doi.org/10.1093/braincomms/fcaa205
Wild CJ, Norton L, Menon DK et al (2022) Disentangling the cognitive, physical, and mental health sequelae of COVID-19. Cell Rep Med. https://doi.org/10.1016/J.XCRM.2022.100750
Miskowiak KW, Johnsen S, Sattler SM et al (2021) Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables. Eur Neuropsychopharmacol 46:39. https://doi.org/10.1016/J.EURONEURO.2021.03.019
Premraj L, Kannapadi N v., Briggs J, et al (2022) Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci 434:120162. https://doi.org/10.1016/J.JNS.2022.120162
Singhavi H, Pai A, Mair M et al (2021) SARS-Cov2: a meta-analysis of symptom distribution by continent in 7310 adult COVID-19 infected patients. Virusdisease 32:400–409. https://doi.org/10.1007/S13337-021-00699-Y/FIGURES/5
Lechien JR, Chiesa-Estomba CM, de Siati DR, et al (2020) Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. European Archives of Oto-Rhino-Laryngology
Mao L, Jin H, Wang M, et al (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2020.1127
Proal AD, VanElzakker MB (2021) Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol 12:. https://doi.org/10.3389/FMICB.2021.698169
Zhou Z, Kang H, Li S, Zhao X (2020) Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol 1. https://doi.org/10.1007/s00415-020-09929-7
Guo P, Benito Ballesteros A, Yeung SP, et al (2022) COVCOG 2: cognitive and memory deficits in long COVID: a second publication from the COVID and Cognition Study. Front Aging Neurosci 14:. https://doi.org/10.3389/FNAGI.2022.804937/FULL
Nasreddine ZS, Phillips NA, Bédirian V et al (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
Ojeda N, del Pino R, Ibarretxe-Bilbao N, et al (2016) [Montreal Cognitive Assessment Test: normalization and standardization for Spanish population]. Rev Neurol 63:488–496. https://doi.org/10.33588/rn.6311.2016241
Wechsler D (2001) Wais III. Escala de inteligencia de wechsler para adultos. Manual de aplicación. TEA Ediciones. Departamento I+D., Barcelona
Schmidt M (1996) Rey Auditory and Verbal Learning Test: A handbook. LosAngeles, CA
Alviarez-Schulze V, Cattaneo G, Pachón-García C, et al (2022) Validation and normative data of the spanish version of the rey auditory verbal learning test and associated long-term forgetting measures in middle-aged adults. Front Aging Neurosci 14:. https://doi.org/10.3389/fnagi.2022.809019
Reitan RMM (1958) Validity of the trail making test as an indicator of organic brain damage. Perceptual and Motos Skills 8:271–276. https://doi.org/10.2466/PMS.8.7.271-276
Lezak, M. D., Howieson, D. B., Bigler, E. D., & Tranel D (2012) Neuropsychological assessment, 5th ed
Benton AL, Hamsher K (1989) Multilingual Aphasia Examination. AJA Associates, Iowa
Peña-Casanova J, Quiñones-Úbeda S, Gramunt-Fombuena N et al (2009) Spanish multicenter normative studies (NEURONORMA Project): norms for verbal fluency tests. Arch Clin Neuropsychol 24:395–411. https://doi.org/10.1093/ARCLIN/ACP042
Ardila A, Ostrosky-Solís F, Bernal B (2006) Cognitive testing toward the future: the example of semantic verbal fluency (ANIMALS). Int J Psychol 41:324–332. https://doi.org/10.1080/00207590500345542
Golden CJ (2005) Test de colores y palabras (Stroop). Madrid
Allegri RF, Mangone CA, Villavicencio AF et al (1997) Spanish boston naming test norms. Clin Neuropsychol 11:416–420. https://doi.org/10.1080/13854049708400471
Fernández-Abascal EG, Cabello R, Fernández-Berrocal P, Baron-Cohen S (2013) Test-retest reliability of the “Reading the Mind in the Eyes” test: A one-year follow-up study. Mol Autism 4:1–6. https://doi.org/10.1186/2040-2392-4-33/TABLES/2
Gomar JJ, Ortiz-Gil J, McKenna PJ et al (2011) Validation of the Word Accentuation Test (TAP) as a means of estimating premorbid IQ in Spanish speakers. Schizophr Res 128:175–176. https://doi.org/10.1016/j.schres.2010.11.016
Jackson C (2015) The Chalder Fatigue Scale (CFQ 11). Occup Med (Chic Ill) 65:86–86. https://doi.org/10.1093/OCCMED/KQU168
García-Campayo J, Zamorano E, Ruiz MA et al (2010) Cultural adaptation into Spanish of the generalized anxiety disorder-7 (GAD-7) scale as a screening tool. Health Qual Life Outcomes 8:8. https://doi.org/10.1186/1477-7525-8-8
Spitzer RL, Kroenke K, Williams JBW, Löwe B (2006) A Brief Measure for Assessing Generalized Anxiety Disorder: The GAD-7. Arch Intern Med 166:1092–1097. https://doi.org/10.1001/ARCHINTE.166.10.1092
Kroenke K, Spitzer RL, Williams JBW (2001) The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 16:606. https://doi.org/10.1046/J.1525-1497.2001.016009606.X
Diez-Quevedo C, Rangil T, Sanchez-Planell L et al (2001) Validation and utility of the patient health questionnaire in diagnosing mental disorders in 1003 general hospital spanish inpatients. Psychosom Med 63:679–686
WHOQOL-BREF (1996) WHOQOL-BREF : introduction, administration, scoring and generic version of the assessment : field trial version, December. World Health Organization 1–16
Marshall JC, Murthy S, Diaz J et al (2020) A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 20:e192–e197. https://doi.org/10.1016/S1473-3099(20)30483-7
Hopkins RO, Weaver LK, Pope D, et al (1999) Neuropsychological Sequelae and Impaired Health Status in Survivors of Severe Acute Respiratory Distress Syndrome
Serrano R, Corbella X, Rello J (2021) Management of hypoxemia in SARS-CoV-2 infection: lessons learned from one year of experience, with a special focus on silent hypoxemia. J Intensive Med 1:26–30. https://doi.org/10.1016/J.JOINTM.2021.02.001
Dhont S, Derom E, van Braeckel E et al (2020) The pathophysiology of “happy” hypoxemia in COVID-19. Respir Res 21:1–9. https://doi.org/10.1186/s12931-020-01462-5
Brugulat-Serrat A, Salvadó G, Operto G et al (2020) White matter hyperintensities mediate gray matter volume and processing speed relationship in cognitively unimpaired participants. Hum Brain Mapp 41:1309. https://doi.org/10.1002/HBM.24877
Penke L, Mañiega SM, Bastin ME, et al (2012) Brain-wide white matter tract integrity is associated with information processing speed and general intelligence. Molecular Psychiatry 2012 17:10 17:955–955. https://doi.org/10.1038/MP.2012.127
Patel SK, Hanly PJ, Smith EE et al (2015) Nocturnal hypoxemia is associated with white matter hyperintensities in patients with a minor stroke or transient ischemic attack. J Clin Sleep Med 11:1417. https://doi.org/10.5664/JCSM.5278
Radnis C, Qiu S, Jhaveri M, et al (2020) Radiographic and clinical neurologic manifestations of COVID-19 related hypoxemia. J Neurol Sci 418:117119. https://doi.org/10.1016/J.JNS.2020.117119
Huang S, Zhou Z, Yang D et al (2022) Persistent white matter changes in recovered COVID-19 patients at the 1-year follow-up. Brain 145:1830–1838. https://doi.org/10.1093/BRAIN/AWAB435
Paniz-Mondolfi A, Bryce C, Grimes Z et al (2020) Central nervous system involvement by severe acute respiratory syndrome coronavirus -2 (SARS-CoV-2). J Med Virol. https://doi.org/10.1002/jmv.25915
Sankowski R, Mader S, Valdés-Ferrer SI (2015) Systemic Inflammation and the Brain: Novel Roles of Genetic, Molecular, and Environmental Cues as Drivers of Neurodegeneration. Front Cell Neurosci 9:. https://doi.org/10.3389/FNCEL.2015.00028
Liddelow SA, Guttenplan KA, Clarke LE, et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017 541:7638 541:481–487. https://doi.org/10.1038/NATURE21029
Wang Z, Yang Y, Liang X, et al (2020) COVID-19 Associated Ischemic Stroke and Hemorrhagic Stroke: Incidence, Potential Pathological Mechanism, and Management. Front Neurol 11:571996. https://doi.org/10.3389/FNEUR.2020.571996
Whiteside DM, Basso MR, Naini SM et al (2022) Outcomes in post-acute sequelae of COVID-19 (PASC) at 6 months post-infection Part 1: Cognitive functioning. Clinical Neuropsychologist 36:806–828. https://doi.org/10.1080/13854046.2022.2030412
Ferrucci R, Dini M, Groppo E et al (2021) Long-lasting cognitive abnormalities after COVID-19. Brain Sci 11:1–11. https://doi.org/10.3390/brainsci11020235
Mattioli F, Stampatori C, Righetti F et al (2021) Neurological and cognitive sequelae of Covid-19: a four month follow-up. J Neurol 268:4422. https://doi.org/10.1007/S00415-021-10579-6
Henneghan AM, Lewis KA, Gill E, Kesler SR (2022) Cognitive Impairment in Non-critical, Mild-to-Moderate COVID-19 Survivors. Front Psychol 13:770459. https://doi.org/10.3389/fpsyg.2022.770459
Lorent N, Weygaerde Y vande, Claeys E, et al (2022) Prospective longitudinal evaluation of hospitalised COVID-19 survivors 3 and 12 months after discharge. ERJ Open Res 8:. https://doi.org/10.1183/23120541.00004-2022
Stavem K, Einvik G, Tholin B, et al (2022) Cognitive function in non-hospitalized patients 8–13 months after acute COVID-19 infection: a cohort study in Norway. PLoS One 17:e0273352. https://doi.org/10.1371/JOURNAL.PONE.0273352
Crunfli F, Carregari VC, Veras FP, et al (2022) Morphological, cellular, and molecular basis of brain infection in COVID-19 patients. Proc Natl Acad Sci U S A 119:. https://doi.org/10.1073/pnas.2200960119
Douaud G, Lee S, Alfaro-Almagro F, et al (2022) SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022 604:7907 604:697–707. https://doi.org/10.1038/s41586-022-04569-5
Boldrini M, Canoll PD, Klein RS (2021) How COVID-19 Affects the Brain. JAMA Psychiat 78:682–683. https://doi.org/10.1001/JAMAPSYCHIATRY.2021.0500
Colbenson GA, Johnson A, Wilson ME (2019) Post-intensive care syndrome: impact, prevention, and management. Breathe 15:98–101. https://doi.org/10.1183/20734735.0013-2019
Walker KA, Ficek BN, Westbrook R (2019) Understanding the role of systemic inflammation in Alzheimer’s disease. ACS Chem Neurosci 10:3340–3342. https://doi.org/10.1021/ACSCHEMNEURO.9B00333/ASSET/IMAGES/LARGE/CN-2019-00333X_0001.JPEG
Butowt R, von Bartheld CS (2022) The route of SARS-CoV-2 to brain infection: have we been barking up the wrong tree? Mol Neurodegener 17:1–4. https://doi.org/10.1186/S13024-022-00529-9/FIGURES/1
Reppucci CJ, Petrovich GD (2015) Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: a single and double retrograde tracing study in rats. Brain Structure and Function 2015 221:6 221:2937–2962. https://doi.org/10.1007/S00429-015-1081-0
Chiappetta S, Sharma AM, Bottino V, Stier C (2020) COVID-19 and the role of chronic inflammation in patients with obesity. Int J Obes 44:1790–1792. https://doi.org/10.1038/s41366-020-0597-4
Chu Y, Yang J, Shi J, et al (2020) Obesity is associated with increased severity of disease in COVID-19 pneumonia: a systematic review and meta-analysis. Eur J Med Res 25:. https://doi.org/10.1186/S40001-020-00464-9
Selim BJ, Ramar K, Surani S (2016) Obe Intensive Care Unit 44:146–156. https://doi.org/10.1080/21548331.2016.1179558
Acknowledgements
This research was supported by the Agency for Management of University and Research Grants (AGAUR) from the Generalitat de Catalunya (Pandemies, 202PANDE00053) and La Marató de TV3 Foundation (202111-30-31-32). NAUTILUS- Project Collaborative Group: Jose A. Bernia and Vanesa Arauzo, Servei d’Anestesia Reanimació i Clinica del Dolor; Servei de Medicina Intensiva. Consorci Sanitari de Terrassa (CST) (Terrassa, Barcelona, Spain). Marta Balague-Marmaña and Berta Valles-Pauls, Hospital Sant Joan Despí Moisès Broggi, Consorci Sanitari Integral (CSI) (sant Joan Despí, Barcelona, Spain); Jesús Caballero, Hospital Universitari Arnau de Vilanova (Lleida, Spain). Anna Carnes-Vendrell and Gerard Piñol-Ripoll, Hospital Universitari de Santa Maria (Lleida, Spain). Ester Gonzalez-Aguado and Carme Tayó-Juli, Consorci Sanitari Alt Penedès-Garraf (Vilafranca de Penedés, Barcelona, Spain). Eva Forcadell-Ferreres and Silvia Reverte-Vilarroya, Hospital Verge de la Cinta, (Tortosa, Tarragona, Spain). Susanna Forné, Fundació Sant Hospital de la Seu d’Urgell (La Seu d’Urgell, Lleida, Spain). Jordina Muñoz-Padros and Anna Bartes-Plan, Consorci Hospitalari de Vic (Vic, Barcelona, Spain). Jose A. Muñoz-Moreno and Anna Prats-Paris, Servei de Malalties Infeccioses, Fundació Lluita contra les Infeccions—Hospital Universitari Germans Trias i Pujol (Badalona, Barcelona, Spain). Inmaculada Rico and Nuria Sabé, Hospital Universitari de Bellvitge (L'Hospitalet de Llobregat, Barcelona, Spain). Laura Casas and Marta Almeria, Hospital Universitari Mútua Terrassa (Terrassa, Barcelona, Spain). Maria José Ciudad and Anna Ferré, Badalona Serveis Assistencials (Badalona, Barcelona, Spain). Manuela Lozano and Tamar Garzon, Institut d'Assistència Sanitària (Girona, Spain). Marta Cullell and Sonia Vega, Fundació Salut Empordà (Figueres, Girona, Spain). Sílvia Alsina, Fundació Hospital de Puigcerdà (Puigcerdà, Girona, Spain). Maria J. Maldonado-Belmonte and Susana Vazquez-Rivera, Hospital Universitario Central de la Cruz Roja San José y Santa Adela (Madrid, Spain). Sandra Navarro and Eva Baillès, Servei Andorrà d'Atenció Sanitària (SAAS) (Andorra).
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Consortia
Contributions
MA, MG, CJ, and BS designed the study. NC and the NAUTILUS Project Collaborative Group collected the data. MA performed the statistical analyses and wrote the first version of the manuscript. CJ revised the manuscript critically for important intellectual content. All authors revised the manuscript drafts and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest, and the authors are responsible for the content and writing of the paper.
Ethical standard statement
The study was conducted with the approval of the Drug Research Ethics Committee (CEIm) of Consorci Sanitari de Terrassa (CEIm code: 02–20-107–070) and the Ethics Committee of the University of Barcelona (IRB00003099).
Patient consent statement
All participants provided written informed consent.
Additional information
NAUTILUS Project Collaborative Group authors are listed in acknowledgement.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ariza, M., Cano, N., Segura, B. et al. COVID-19 severity is related to poor executive function in people with post-COVID conditions. J Neurol 270, 2392–2408 (2023). https://doi.org/10.1007/s00415-023-11587-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11587-4