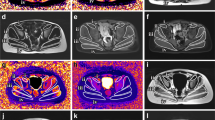
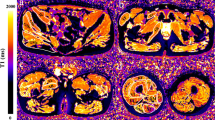Abstract
Objective
To describe the disease progression of Duchenne muscular dystrophy (DMD) in the pelvic and thigh muscles over 1-year using multiple-parameter quantitative magnetic resonance imaging (qMRI), and to determine the most responsive muscle and predict subclinical disease progression in functionally stable patients.
Methods
Fifty-four DMD patients (mean age 8.9 ± 2.5, range 5–15 years) completed baseline and 1-year follow-up qMRI examinations/biomarkers [3-point Dixon/fat fraction (FF); T1 mapping/T1; T2 mapping/T2]. Meanwhile, clinical assessments [NorthStar ambulatory assessment (NSAA) score] and timed function tests were performed in DMD patients. Twenty-four healthy male controls (range 5–15 years) accomplished baseline qMRI examinations. Group differences were compared using the Wilcoxon test. The standardized response mean (SRM) was taken as the responsiveness to the disease progression index.
Results
FF, T1, and T2 in all DMD age subgroups changed significantly over 1-year (P < 0.05). Even in functionally stable patients (NSAA score increased, unchanged, or decreased by 1-point) over 1-year, significant increases in FF and T2 and decreases in T1 were observed in gluteus maximus (GMa), gluteus medius, vastus lateralis, and adductor magnus (P < 0.05). Overall, the SRM of FF, T1, and T2 was all the highest in GMa, which were 1.25, − 0.92, and 0.93, respectively.
Conclusions
qMRI biomarkers are responsive to disease progression and can also detect subclinical disease progression in functionally stable DMD patients over 1-year. GMa is the most responsive to disease progression of all the muscles analyzed.
Trial registration
Chinese Clinical Trial Registry (http://www.chictr.org.cn/index.aspx) ChiCTR1800018340, 09/12/2018, prospectively registered.






Similar content being viewed by others
References
Duan D, Goemans N, Takeda S, Mercuri E, Aartsma-Rus A (2021) Duchenne muscular dystrophy. Nat Rev Dis Primers 7:13. https://doi.org/10.1038/s41572-021-00248-3
Mercuri E, Bönnemann CG, Muntoni F (2019) Muscular dystrophies. Lancet 394:2025–2038. https://doi.org/10.1016/s0140-6736(19)32910-1
Birnkrant DJ, Bello L, Butterfield RJ et al (2022) Cardiorespiratory management of Duchenne muscular dystrophy: emerging therapies, neuromuscular genetics, and new clinical challenges. Lancet Respir Med 10:403–420. https://doi.org/10.1016/s2213-2600(21)00581-6
Tsuda T (2018) Clinical Manifestations and overall management strategies for Duchenne muscular dystrophy. Methods Mol Biol 1687:19–28. https://doi.org/10.1007/978-1-4939-7374-3_2
Nascimento Osorio A, Medina Cantillo J, Camacho Salas A, Madruga Garrido M, Vilchez Padilla JJ (2019) Consensus on the diagnosis, treatment and follow-up of patients with Duchenne muscular dystrophy. Neurologia (Engl Ed). 34:469–481. https://doi.org/10.1016/j.nrl.2018.01.001
Dahlqvist JR, Widholm P, Leinhard OD, Vissing J (2020) MRI in neuromuscular diseases: an emerging diagnostic tool and biomarker for prognosis and efficacy. Ann Neurol 88:669–681. https://doi.org/10.1002/ana.25804
Hogrel JY, Wary C, Moraux A et al (2016) Longitudinal functional and NMR assessment of upper limbs in Duchenne muscular dystrophy. Neurology 86:1022–1030. https://doi.org/10.1212/wnl.0000000000002464
Willcocks RJ, Rooney WD, Triplett WT et al (2016) Multicenter prospective longitudinal study of magnetic resonance biomarkers in a large duchenne muscular dystrophy cohort. Ann Neurol 79:535–547. https://doi.org/10.1002/ana.24599
Barnard AM, Willcocks RJ, Triplett WT et al (2020) MR biomarkers predict clinical function in Duchenne muscular dystrophy. Neurology 94:e897–e909. https://doi.org/10.1212/wnl.0000000000009012
Nagy S, Schädelin S, Hafner P et al (2020) Longitudinal reliability of outcome measures in patients with Duchenne muscular dystrophy. Muscle Nerve 61:63–68. https://doi.org/10.1002/mus.26690
Kim HK, Laor T, Horn PS, Racadio JM, Wong B, Dardzinski BJ (2010) T2 mapping in Duchenne muscular dystrophy: distribution of disease activity and correlation with clinical assessments. Radiology 255:899–908. https://doi.org/10.1148/radiol.10091547
Godi C, Ambrosi A, Nicastro F et al (2016) Longitudinal MRI quantification of muscle degeneration in Duchenne muscular dystrophy. Ann Clin Transl Neurol 3:607–622. https://doi.org/10.1002/acn3.319
Marden FA, Connolly AM, Siegel MJ, Rubin DA (2005) Compositional analysis of muscle in boys with Duchenne muscular dystrophy using MR imaging. Skelet Radiol. 34:140–148. https://doi.org/10.1007/s00256-004-0825-3
Mercuri E, Pichiecchio A, Allsop J, Messina S, Pane M, Muntoni F (2007) Muscle MRI in inherited neuromuscular disorders: past, present, and future. J Magn Reson Imaging 25:433–440. https://doi.org/10.1002/jmri.20804
Kim HK, Merrow AC, Shiraj S, Wong BL, Horn PS, Laor T (2013) Analysis of fatty infiltration and inflammation of the pelvic and thigh muscles in boys with Duchenne muscular dystrophy (DMD): grading of disease involvement on MR imaging and correlation with clinical assessments. Pediatr Radiol 43:1327–1335. https://doi.org/10.1007/s00247-013-2696-z
Peng F, Xu H, Song Y et al (2022) Utilization of T1-mapping for the pelvic and thigh muscles in Duchenne muscular dystrophy: a quantitative biomarker for disease involvement and correlation with clinical assessments. BMC Musculoskelet Disord 23:681. https://doi.org/10.1186/s12891-022-05640-y
Ropars J, Gravot F, Ben Salem D, Rousseau F, Brochard S, Pons C (2020) Muscle MRI: a biomarker of disease severity in Duchenne muscular dystrophy? A systematic review. Neurology 94:117–133. https://doi.org/10.1212/wnl.0000000000008811
Bushby K, Connor E (2011) Clinical outcome measures for trials in Duchenne muscular dystrophy: report from International Working Group meetings. Clin Investig (Lond). 1:1217–1235. https://doi.org/10.4155/cli.11.113
McDonald CM, Henricson EK, Abresch RT et al (2013) The 6-minute walk test and other endpoints in Duchenne muscular dystrophy: longitudinal natural history observations over 48 weeks from a multicenter study. Muscle Nerve 48:343–356. https://doi.org/10.1002/mus.23902
Scott E, Eagle M, Mayhew A et al (2012) Development of a functional assessment scale for ambulatory boys with Duchenne muscular dystrophy. Physiother Res Int 7:101–109. https://doi.org/10.1002/pri.520
Ricotti V, Ridout DA, Pane M et al (2016) The NorthStar Ambulatory Assessment in Duchenne muscular dystrophy: considerations for the design of clinical trials. J Neurol Neurosurg Psychiatry 87:149–155. https://doi.org/10.1136/jnnp-2014-309405
Marty B, Carlier PG (2019) Physiological and pathological skeletal muscle T1 changes quantified using a fast inversion-recovery radial NMR imaging sequence. Sci Rep 9:6852. https://doi.org/10.1038/s41598-019-43398-x
Fu C, Xia Y, Meng F et al (2020) MRI quantitative analysis of eccentric exercise-induced skeletal muscle injury in rats. Acad Radiol 27:e72–e79. https://doi.org/10.1016/j.acra.2019.05.011
Muntoni F, Domingos J, Manzur AY et al (2019) Categorising trajectories and individual item changes of the North Star Ambulatory Assessment in patients with Duchenne muscular dystrophy. PLoS ONE 14:e0221097. https://doi.org/10.1371/journal.pone.0221097
Morrow JM, Sinclair CD, Fischmann A et al (2016) MRI biomarker assessment of neuromuscular disease progression: a prospective observational cohort study. Lancet Neurol 15:65–77. https://doi.org/10.1016/s1474-4422(15)00242-2
Liang MH, Fossel AH, Larson MG (1990) Comparisons of five health status instruments for orthopedic evaluation. Med Care 28:632–642. https://doi.org/10.1097/00005650-199007000-00008
Bonati U, Hafner P, Schädelin S et al (2015) Quantitative muscle MRI: a powerful surrogate outcome measure in Duchenne muscular dystrophy. Neuromuscul Disord 25:679–685. https://doi.org/10.1016/j.nmd.2015.05.006
Mankodi A, Bishop CA, Auh S, Newbould RD, Fischbeck KH, Janiczek RL (2016) Quantifying disease activity in fatty-infiltrated skeletal muscle by IDEAL-CPMG in Duchenne muscular dystrophy. Neuromuscul Disord 26:650–658. https://doi.org/10.1016/j.nmd.2016.07.013
Polavarapu K, Manjunath M, Preethish-Kumar V et al (2016) Muscle MRI in Duchenne muscular dystrophy: evidence of a distinctive pattern. Neuromuscul Disord 26:768–774. https://doi.org/10.1016/j.nmd.2016.09.002
Leung DG (2019) Advancements in magnetic resonance imaging-based biomarkers for muscular dystrophy. Muscle Nerve 60:347–360. https://doi.org/10.1002/mus.26497
Araujo ECA, Marty B, Carlier PG, Baudin PY, Reyngoudt H (2021) Multiexponential analysis of the water T2-relaxation in the skeletal muscle provides distinct markers of disease activity between inflammatory and dystrophic myopathies. J Magn Reson Imaging 53(1):181–189. https://doi.org/10.1002/jmri.27300
Bulluck H, Maestrini V, Rosmini S et al (2015) Myocardial T1 mapping. Circ J 79:487–494. https://doi.org/10.1253/circj.CJ-15-0054
Liu CY, Yao J, Kovacs WC et al (2021) Skeletal muscle magnetic resonance biomarkers in GNE myopathy. Neurology 96:e798–e808. https://doi.org/10.1212/wnl.0000000000011231
Sherlock SP, Zhang Y, Binks M, Marraffino S (2021) Quantitative muscle MRI biomarkers in Duchenne muscular dystrophy: cross-sectional correlations with age and functional tests. Biomark Med 15:761–773. https://doi.org/10.2217/bmm-2020-0801
Funding
This work was supported by National Natural Science Foundation of China (Grand Nos. 81860316, 81971586, 82071874, 81901712, 821201080105); Jiangxi Provincial key Natural Science Foundation of China (20212ACB206021); Sichuan Science and Technology Program (21ZDYF1967); Clinical Research Finding of Chinese Society of Cardiovascular Disease (CSC) of 2019 (No. HFCSC2019B01); and Fellowship of China postdoctoral science foundation (2021M692290).
Author information
Authors and Affiliations
Contributions
FP and HX participated in the study design, contributed to data analysis and interpretation, statistical analysis, and drafted the main manuscript, and are the co-first authors. YS and KX carried out data acquisition and contributed to image quality controlling. SL contributed to editing and review of the manuscript. XC, YG, and LG participated in the whole study design, contributed to quality control of data and algorithms, editing and review of the manuscript, and are the co-corresponding authors. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standard statement
This study was approved by the local Institutional Review Board and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Written informed consent was acquired from all participants and guardians before study participation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, F., Xu, H., Song, Y. et al. Longitudinal study of multi-parameter quantitative magnetic resonance imaging in Duchenne muscular dystrophy: hyperresponsiveness of gluteus maximus and detection of subclinical disease progression in functionally stable patients. J Neurol 270, 1439–1451 (2023). https://doi.org/10.1007/s00415-022-11470-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11470-8