Abstract
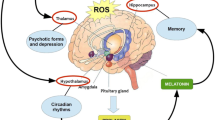
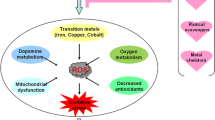
Parkinson’s disease (PD) is an ageing disorder with deterioration of dopamine neurons which leads to motor complications like tremor, stiffness, slow movement and postural disturbances. In PD, both genetics as well as environmental factors both play a major role in causing the pathogenesis. Though there are surfeit of risk factors involved in PD occurrence, till now there is lack of an exact causative agent as a risk for PD with confirmative findings. The role of heavy metals reported to be a significant factor in PD pathogenesis. Heavy metal functions in cell maintenance but growing pieces of evidences reported to cause dyshomeostasis with increased PD rate. Metals disturb the molecular processes and results in oxidative stress, DNA damage, mitochondrial dysfunction, and apoptosis. The present review elucidates the role of cobalt, nickel, mercury, chromium, thallium metals in α-synuclein aggregation and its involvement in blood brain barrier flux. Also, the review explains the plausible role of aforementioned metals with a mechanistic approach and therapeutic recommendations in PD.





Similar content being viewed by others
Availability of data and material
Not applicable.
References
Venkatesan D, Iyer M, Krishnan P et al (2021) A late-onset Parkinson’s disease in tribes in India–a case report. Brain Disord 3:100015
Venkatesan D, Iyer M, Narayanasamy A et al (2020) Kynurenine pathway in Parkinson’s disease—an update. Eneurologicalsci 21:100270
Venkatesan D, Iyer M, Narayanasamy A et al (2022) Genotypic-phenotypic analysis, metabolic profiling and clinical correlations in Parkinson’s disease patients from Tamil Nadu population, India. J Mol Neurosci. https://doi.org/10.1007/s12031-022-02028-4
Iyer M, Subramaniam MD, Venkatesan D et al (2021) Role of RhoA-ROCK signaling in Parkinson’s disease. Eur J Pharmacol 894:173815
Jayaramayya K, Iyer M, Venkatesan D et al (2020) Unraveling correlative roles of dopamine transporter (DAT) and Parkin in Parkinson’s disease (PD): a road to discovery? Brain Res Bull 157:169–179
Mohana Devi S, Mahalaxmi I, Aswathy NP et al (2020) Does retina play a role in Parkinson’s disease? Acta Neurol Belg 120:257–265
Mahalaxmi I, Subramaniam MD, Gopalakrishnan AV, Vellingiri B (2021) Dysfunction in mitochondrial electron transport chain complex I, Pyruvate Dehydrogenase Activity, And Mutations in ND1 and ND4 gene in autism spectrum disorder subjects from Tamil Nadu population, India. Mol Neurobiol 58:5303–5311
Vellingiri B, Suriyanarayanan A, Selvaraj P et al (2022) Role of heavy metals (copper (Cu), arsenic (As), cadmium (Cd), iron (Fe) and lithium (Li)) induced neurotoxicity. Chemosphere 301:134625
Green AJ, Planchart A (2018) The neurological toxicity of heavy metals: a fish perspective. Comp Biochem Physiol Part C 208:12–19
Chen P, Miah MR, Aschner M (2016) Metals and neurodegeneration. F1000Research 5:366
Raj K, Kaur P, Gupta G, Singh S (2021) Metals associated neurodegeneration in Parkinson’s disease: Insight to physiological, pathological mechanisms and management. Neurosci Lett 753:135873
Bjorklund G, Stejskal V, Urbina MA et al (2018) Metals and Parkinson’s disease: mechanisms and biochemical processes. Curr Med Chem 25:2198–2214
Piao Y-S, Lian T-H, Hu Y et al (2017) Restless legs syndrome in Parkinson disease: clinical characteristics, abnormal iron metabolism and altered neurotransmitters. Sci Rep 7:1–10
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6:e04691
Engwa GA, Ferdinand PU, Nwalo FN, Unachukwu MN (2019) Mechanism and health effects of heavy metal toxicity in humans. Poisoning Mod World-New Tricks Old Dog. https://doi.org/10.5772/intechopen.82511
Singh N, Sharma B (2021) On the mechanisms of heavy metal-induced neurotoxicity: amelioration by plant products. Proc Natl Acad Sci India Sect B Biol Sci. https://doi.org/10.1007/s40011-021-01272-9
Caito S, Aschner M (2015) Neurotoxicity of metals. Handb Clin Neurol 131:169–189
Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev 4:118
Ijomone OM, Ifenatuoha CW, Aluko OM, Ijomone OK, Aschner M (2020) The aging brain: impact of heavy metal neurotoxicity. Crit Rev Toxicol 50(9):801–814
Kumar A, Singh N, Pandey R et al (2018) Biochemical and molecular targets of heavy metals and their actions. Biomedical applications of metals. Springer, Berlin, pp 297–319
Zheng W, Aschner M, Ghersi-Egea J-F (2003) Brain barrier systems: a new frontier in metal neurotoxicological research. Toxicol Appl Pharmacol 192:1–11
Bose-O’Reilly S, McCarty KM, Steckling N, Lettmeier B (2010) Mercury exposure and children’s health. Curr Probl Pediatr Adolesc Health Care 40:186–215
Chang L, Shen S, Zhang Z et al (2018) Study on the relationship between age and the concentrations of heavy metal elements in human bone. Ann Transl Med 6:320
Magnus MM, T, (2006) Ageing and neuronal vulnerability. Nat Rev Neurosci 7:278–294
Mattson MP, Arumugam TV (2018) Hallmarks of brain aging: adaptive and pathological modification by metabolic states. Cell Metab 27:1176–1199
Pamphlett R, Bishop DP (2022) Mercury is present in neurons and oligodendrocytes in regions of the brain affected by Parkinson’s disease and co-localises with Lewy bodies. PLoS ONE 17(1):e0262464
He ZL, Yang XE, Stoffella PJ (2005) Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol 19:125–140
Bradl H (2005) Heavy metals in the environment: origin, interaction and remediation. Elsevier, Amsterdam
Fergusson JE (1990) The heavy elements: chemistry, environmental impact and health effects. Oxford, England
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants, 3rd edn. Boca Raton, CRC Press
Liu F, Mahmood M, Xu Y et al (2015) Effects of silver nanoparticles on human and rat embryonic neural stem cells. Front Neurosci 9:115
Geffroy B, Ladhar C, Cambier S et al (2012) Impact of dietary gold nanoparticles in zebrafish at very low contamination pressure: the role of size, concentration and exposure time. Nanotoxicology 6:144–160
Xue YJ, Wu Y, Sun J (2012) Four types inorg nanoparticles stimul inflamm react brain microglia damage neurons. Vitro Toxicol Lett 214:91–98
Xie Y, Wang Y, Zhang T et al (2012) Effects of nanoparticle zinc oxide on spatial cognition and synaptic plasticity in mice with depressive-like behaviors. J Biomed Sci 19:1–11
Czajka M, Sawicki K, Sikorska K et al (2015) Toxicity of titanium dioxide nanoparticles in central nervous system. Toxicol In Vitro 29:1042–1052
Matysiak M, Kapka-Skrzypczak L, Brzóska K et al (2016) Proteomic approach to nanotoxicity. J Proteom 137:35–44
Singh SP, Rahman M, Murty U et al (2013) Comparative study of genotoxicity and tissue distribution of nano and micron sized iron oxide in rats after acute oral treatment. Toxicol Appl Pharmacol 266:56–66
Oszlánczi G, Vezér T, Sárközi L et al (2010) Functional neurotoxicity of Mn-containing nanoparticles in rats. Ecotoxicol Environ Saf 73:2004–2009
Papp A, Oszlánczi G, Horváth E et al (2012) Consequences of subacute intratracheal exposure of rats to cadmium oxide nanoparticles: electrophysiological and toxicological effects. Toxicol Ind Health 28:933–941
Sticker CB, Story MC (2008) The mercury detox & amalgam fillings forum detoxing heavy metals, removing amalgam fillings, understanding mercury poisoning our most popular videos, audio clips, and articles. J Neuroimmune Pharmacol 3:286–295
Zhao J, Xu L, Zhang T et al (2009) Influences of nanoparticle zinc oxide on acutely isolated rat hippocampal CA3 pyramidal neurons. Neurotoxicology 30:220–230
De Simone U, Roccio M, Gribaldo L et al (2018) Human 3D cultures as models for evaluating magnetic nanoparticle CNS cytotoxicity after short-and repeated long-term exposure. Int J Mol Sci 19:1993
Hong F, Zhou Y, Ji J et al (2018) Nano-TiO2 inhibits development of the central nervous system and its mechanism in offspring mice. J Agric Food Chem 66:11767–11774
Liu H, Ma L, Zhao J et al (2009) Biochemical toxicity of nano-anatase TiO2 particles in mice. Biol Trace Elem Res 129:170–180
Hardas SS, Butterfield DA, Sultana R et al (2010) Brain distribution and toxicological evaluation of a systemically delivered engineered nanoscale ceria. Toxicol Sci 116:562–576
Wang B, Feng WY, Wang M et al (2007) Transport of intranasally instilled fine Fe2O3 particles into the brain: micro-distribution, chemical states, and histopathological observation. Biol Trace Elem Res 118:233–243
Lucchini R, Dorman D, Elder A, Veronesi B (2012) Neurological impacts from inhalation of pollutants and the nose–brain connection. Neurotoxicol 33:838–841
Takács S, Szabó A, Oszlánczi G et al (2012) Repeated simultaneous cortical electrophysiological and behavioral recording in rats exposed to manganese-containing nanoparticles. Acta Biol Hung 63:426–440
Sárközi L, Horváth E, Kónya Z et al (2009) Subacute intratracheal exposure of rats to manganese nanoparticles: behavioral, electrophysiological, and general toxicological effects. Inhal Toxicol 21:83–91
Sawicki K, Czajka M, Matysiak-Kucharek M et al (2019) Toxicity of metallic nanoparticles in the central nervous system. Nanotechnol Rev 8(1):175–200
Han D, Tian Y, Zhang T, Ren G, Yang Z (2011) Nano-zinc oxide damages spatial cognition capability via over-enhanced long-term potentiation in hippocampus of Wistar rats. Int J Nanomedicine 6:1453–1461
Xu F, Piett C, Farkas S, Qazzaz M, Syed NI (2013) Silver nanoparticles (AgNPs) cause degeneration of cytoskeleton and disrupt synaptic machinery of cultured cortical neurons. Mol Brain 19:6–29
Wang J, Rahman MF, Duhart HM et al (2009) Expression changes of dopaminergic system-related genes in PC12 cells induced by manganese, silver, or copper nanoparticles. Neurotoxicology 30(6):926–933
Wardlaw JM, Smith EE, Biessels GJ et al (2013) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12:822–838
Lee C, Cho S-D, Chang D-S et al (2006) Food safety guidelines for consumer. Safe Food 1:31–43
Wang XF, Xing ML, Shen Y, Zhu X, Xu LH (2006) Oral administration of Cr (VI) induced oxidative stress, DNA damage and apoptotic cell death in mice. Toxicology 228:16–23
Saraiva C, Praça C, Ferreira R et al (2016) Nanoparticle-mediated brain drug delivery: overcoming blood–brain barrier to treat neurodegenerative diseases. J Controll Release 235:34–47
Obermeier B, Daneman R, Ransohoff RM (2013) Development, maintenance and disruption of the blood-brain barrier. Nat Med 19:1584–1596
Mozaffarian D, Benjamin EJ, Go AS et al (2015) Heart disease and stroke statistics update: a report from the American Heart Association. Circulation 131:e29–e322
Abbott NJ, Patabendige AA, Dolman DE et al (2010) Structure and function of the blood–brain barrier. Neurobiol Dis 37:13–25
Kolhar P, Anselmo AC, Gupta V et al (2013) Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. Proc Natl Acad Sci 110:10753–10758
Ximenes-da-Silva A (2016) Metal ion toxins and brain aquaporin-4 expression: an overview. Front Neurosci 10:233
Wang B, Cui Z, Zhong Z et al (2015) Curcumin attenuates brain edema in mice with intracerebral hemorrhage through inhibition of AQP4 and AQP9 expression. Acta Pharmacol Sin 36:939–948
Uversky VN, Li J, Fink AL (2001) Metal-triggered structural transformations, aggregation, and fibrillation of human α-synuclein: a possible molecular link between Parkinson’s disease and heavy metal exposure. J Biol Chem 276:44284–44296
Binolfi A, Rasia RM, Bertoncini CW et al (2006) Interaction of α-synuclein with divalent metal ions reveals key differences: A link between structure, binding specificity and fibrillation enhancement. J Am Chem Soc 128:9893–9901
Bisaglia M, Tessari I, Mammi S, Bubacco L (2009) Interaction between α-synuclein and metal ions, still looking for a role in the pathogenesis of Parkinson’s disease. Neuromolecular Med 11:239–251
Lowe R, Pountney DL, Jensen PH et al (2004) Calcium (II) selectively induces α-synuclein annular oligomers via interaction with the C-terminal domain. Protein Sci 13:3245–3252
Lee C, Hu M (2021) Parkinsonism in a patient with metal on metal total hip replacement related elevated serum heavy metal levels. Cureus. https://doi.org/10.7759/cureus.17791
Wongkongkathep P, Han JY, Choi TS et al (2018) Native top-down mass spectrometry and ion mobility MS for characterizing the cobalt and manganese metal binding of α-synuclein protein. J Am Soc Mass Spectrom 29:1870–1880
de Pomerai DI, Iqbal N, Lafayette I et al (2016) Microwave fields have little effect on α-synuclein aggregation in a Caenorhabditis elegans model of Parkinson’s disease. Bioelectromagnetics 37:116–129
Lee J-H, Choi S-H, Baek M-W et al (2013) CoCl2 induces apoptosis through the mitochondria-and death receptor-mediated pathway in the mouse embryonic stem cells. Mol Cell Biochem 379:133–140
Chen J-X, Zhao T, Huang D-X (2009) Protective effects of edaravone against cobalt chloride-induced apoptosis in PC12 cells. Neurosci Bull 25:67–74
Jung K-H, Chu K, Lee S-T et al (2008) Circulating endothelial progenitor cells as a pathogenetic marker of moyamoya disease. J Cereb Blood Flow Metab 28:1795–1803
Zheng F, Li Y, Zhang F et al (2021) Cobalt induces neurodegenerative damages through Pin1 inactivation in mice and human neuroglioma cells. J Hazard Mater 419:126378
Li D, Liu Z, Chen W et al (2014) Association of glycogen synthase kinase-3β with Parkinson’s disease. Mol Med Rep 9:2043–2050
He MD, Xu SC, Lu YH et al (2011) L-Carnitine protects against nickel-induced neurotoxicity by maintaining mitochondrial function in neuro-2a cells. Toxicol Appl Pharmacol 253(1):38–44
Henriksson J, Tallkvist J, Tjälve H (1997) Uptake of nickel into the brain via olfactory neurons in rats. Toxicol Lett 91:153–162
Ijomone OM (2021) Neurotoxicity of nickel. Advances in neurotoxicology. Elsevier, Amsterdam, pp 263–284
Ali SF, Hasan M, Anwar J (1980) Effect of nickel on the levels of dopamine, noradenaline and serotonin in different regions of the rat brain. Acta Pharmacol Toxicol Copenh 47:318–319
Lamtai M, Chaibat J, Ouakki S et al (2018) Effect of chronic administration of nickel on affective and cognitive behavior in male and female rats: possible implication of oxidative stress pathway. Brain Sci 8:141
Slotkin TA, Seidler FJ (2009) Oxidative and excitatory mechanisms of developmental neurotoxicity: transcriptional profiles for chlorpyrifos, diazinon, dieldrin, and divalent nickel in PC12 cells. Environ Health Perspect 117:587–596
Xu S, He M, Zhong M et al (2010) Melatonin protects against Nickel-induced neurotoxicity in vitro by reducing oxidative stress and maintaining mitochondrial function. J Pineal Res 49:86–94
Uppala R, McKinney RW, Brant KA et al (2015) Nickel inhibits mitochondrial fatty acid oxidation. Biochem Biophys Res Commun 463:806–810
Ijomone OM, Olatunji SY, Owolabi JO et al (2018) Nickel-induced neurodegeneration in the hippocampus, striatum and cortex; an ultrastructural insight, and the role of caspase-3 and α-synuclein. J Trace Elem Med Biol 50:16–23
Ijomone OM, Okori SO, Ijomone OK, Ebokaiwe AP (2018) Sub-acute nickel exposure impairs behavior, alters neuronal microarchitecture, and induces oxidative stress in rats’ brain. Drug Chem Toxicol 41:377–384
Topal A, Atamanalp M, Oruç E et al (2015) Neurotoxic effects of nickel chloride in the rainbow trout brain: assessment of c-Fos activity, antioxidant responses, acetylcholinesterase activity, and histopathological changes. Fish Physiol Biochem 41:625–634
Gopal R, Narmada S, Vijayakumar R, Jaleel CA (2009) Chelating efficacy of CaNa2 EDTA on nickel-induced toxicity in Cirrhinus mrigala (Ham.) through its effects on glutathione peroxidase, reduced glutathione and lipid peroxidation. C R Biol 332:685–696
Reena N, Deepti P, Shruti K (2012) Markers of oxidative stress in generalized anxiety psychiatric disorder: therapeutic implications. J Stress Physiol Biochem 8:32–38
Song X, Kenston SSF, Kong L, Zhao J (2017) Molecular mechanisms of nickel induced neurotoxicity and chemoprevention. Toxicology 392:47–54
Kubrak OI, Husak VV, Rovenko BM et al (2013) Antioxidant system efficiently protects goldfish gills from Ni2+-induced oxidative stress. Chemosphere 90:971–976
Adedara IA, Adegbosin AN, Abiola MA et al (2020) Neurobehavioural and biochemical responses associated with exposure to binary waterborne mixtures of zinc and nickel in rats. Environ Toxicol Pharmacol 73:103294
Brant KA, Fabisiak JP (2009) Nickel and the microbial toxin, MALP-2, stimulate proangiogenic mediators from human lung fibroblasts via a HIF-1α and COX-2–mediated pathway. Toxicol Sci 107:227–237
Kang GS, Li Q, Chen H, Costa M (2006) Effect of metal ions on HIF-1α and Fe homeostasis in human A549 cells. Mutat Res Toxicol Environ Mutagen 610:48–55
Pietruska JR, Liu X, Smith A et al (2011) Bioavailability, intracellular mobilization of nickel, and HIF-1α activation in human lung epithelial cells exposed to metallic nickel and nickel oxide nanoparticles. Toxicol Sci 124:138–148
Balachandar V, Arun M, Mohana Devi S et al (2010) Evaluation of the genetic alterations in direct and indirect exposures of hexavalent chromium [Cr (VI)] in leather tanning industry workers North Arcot District, South India. Int Arch Occup Environ Health 83:791–801
Holland SL, Avery SV (2011) Chromate toxicity and the role of sulfur. Metallomics 3:1119–1123
Guttmann D, Poage G, Johnston T, Zhitkovich A (2008) Reduction with glutathione is a weakly mutagenic pathway in chromium (VI) metabolism. Chem Res Toxicol 21:2188–2194
Macfie A, Hagan E, Zhitkovich A (2010) Mechanism of DNA− protein cross-linking by chromium. Chem Res Toxicol 23:341–347
Azar J, Yousef MH, El-Fawal HA, Abdelnaser A (2021) Mercury and Alzheimer’s disease: a look at the links and evidence. Metab Brain Dis 36:361–374
Osorio-Rico L, Santamaria A, Galván-Arzate S (2017) Thallium toxicity: general issues, neurological symptoms, and neurotoxic mechanisms. Neurotoxicity of metals. Springer, Berlin, pp 345–353
Nava-Ruíz C, Méndez-Armenta M (2013) Cadmium, lead, thallium: occurrence, neurotoxicity and histopathological changes of the nervous system. Pollutant diseases, remediation and recycling. Springer, Berlin, pp 321–349
Osorio-Rico L, Santamaría A, Ali SF, Galván-Arzate S (2021) Neurotoxicity of thallium: old issues and new developments. Advances in neurotoxicology. Elsevier, Amsterdam, pp 285–297
Troisi J, Landolfi A, Cavallo P et al (2021) Metabolomics in Parkinson’s disease. Advances in clinical chemistry. Elsevier, Amstterdam, pp 107–149
Forte G, Alimonti A, Pino A et al (2005) Metals and oxidative stress in patients with Parkinson’s disease. Ann DellIstituto Super Sanità 41:189–195
Aguilar M, Jiménez-Jiménez F, Molina J et al (1998) Cerebrospinal fluid selenium and chromium levels in patients with Parkinson’s disease. J Neural Transm 105:1245–1251
Sanyal J, Ahmed SS, Ng HKT et al (2016) Metallomic biomarkers in cerebrospinal fluid and serum in patients with Parkinson’s disease in Indian population. Sci Rep 6:1–11
Gellein K, Syversen T, Steinnes E et al (2008) Trace elements in serum from patients with Parkinson’s disease—a prospective case-control study. Brain Res 1219:111–115
Lucio M, Willkommen D, Schroeter M et al (2019) Integrative metabolomic and metallomic analysis in a case–control cohort with Parkinson’s disease. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2019.00331
Maass F, Michalke B, Leha A et al (2018) Elemental fingerprint as a cerebrospinal fluid biomarker for the diagnosis of Parkinson’s disease. J Neurochem 145:342–351
Tosato M, Di Marco V (2019) Metal chelation therapy and Parkinson’s disease: a critical review on the thermodynamics of complex formation between relevant metal ions and promising or established drugs. Biomolecules 9:269
Kim J-J, Kim Y-S, Kumar V (2019) Heavy metal toxicity: an update of chelating therapeutic strategies. J Trace Elem Med Biol 54:226–231
Kosnett MJ (2013) The role of chelation in the treatment of arsenic and mercury poisoning. J Med Toxicol 9:347–354
Sunderman F (1978) Clinical response to therapeutic agents in poisoning from mercury vapor. Ann Clin Lab Sci 8:259–269
Banner W Jr, Koch M, Capin D et al (1986) Experimental chelation therapy in chromium, lead, and boron intoxication with N-acetylcysteine and other compounds. Toxicol Appl Pharmacol 83:142–147
Kristensen ME (1981) Toxic hepatitis induced by disulfiram in a non-alcoholic. Acta Med Scand 209:335–336
Wainwright A, Kox W, House I et al (1988) Clinical features and therapy of acute thallium poisoning. QJM Int J Med 69:939–944
Smith SW (2013) The role of chelation in the treatment of other metal poisonings. J Med Toxicol 9:355–369
Acknowledgements
The authors would like to thank Indian Council of Medical Research (ICMR), Department of Health Research—Grant-In-Aid (DHR-GIA) (grant number: GIA/2019/000276/PRCGIA), Government of India and Bharathiar University, Coimbatore, to carry out this research work.
Funding
This work was supported by the Indian Council of Medical Research DHR-GIA [grant number: GIA/2019/000276/PRCGIA], Government of India.
Author information
Authors and Affiliations
Contributions
Conceptualization was done by BV; Data curation was done by AS, KSA, NR, DV, and MI; Funding Acquisition was done by BV; Investigation was done by DV and MI; Roles/Writing–original draft were done by AS, KSA, NR, DV, and MI; Resources were done by DV and MI; Supervision was done by BV and AVG.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.
Consent for publication
All authors have approved for publication.
Rights and permissions
About this article
Cite this article
Vellingiri, B., Suriyanarayanan, A., Abraham, K.S. et al. Influence of heavy metals in Parkinson’s disease: an overview. J Neurol 269, 5798–5811 (2022). https://doi.org/10.1007/s00415-022-11282-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11282-w