Abstract
Background
There is increasing evidence for the role of inflammation in the pathogenesis of mitochondrial diseases (MDs). However, the mechanisms underlying mutation-induced inflammation in MD remain elusive. Our previous study suggested that mitophagy is impaired in the skeletal muscle of those with MD, likely causing mitochondrial DNA (mtDNA) release and thereby triggering inflammation. We here aimed to decipher the role of the cGAS-STING pathway in inflammatory process in MDs.
Methods
We investigated the levels of circulating cell-free mtDNA (ccf-mtDNA) in the serum of 104 patients with MDs. Immunofluorescence was performed in skeletal muscles in MDs and control. Biochemical analysis of muscle biopsies was conducted with western blot to detect cGAS, STING, TBK1, IRF3 and phosphorylated IRF3 (p-IRF3). RT-qPCR was performed to detect the downstream genes of type I interferon in skeletal muscles. Furthermore, a protein microarray was used to examine the cytokine levels in the serum of patients with MDs.
Results
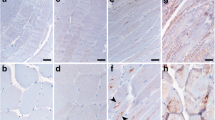
We found that ccf-mtDNA levels were significantly increased in those with MDs compared to the controls. Consistently, the immunofluorescent results showed that cytosolic dsDNA levels were increased in the muscle samples of MD patients. Biochemical analysis of muscle biopsies showed that cGAS, IRF3, and TBK1 protein levels were significantly increased in those with MDs, indicating that there was activation of the cGAS-STING pathway. RT-qPCR showed that downstream genes of type I interferon were upregulated in muscle samples of MDs. Protein microarray results showed that a total of six cytokines associated with the cGAS-STING pathway were significantly increased in MD patients (fold change > 1.2, p value < 0.05).
Conclusions
These findings suggest that increases in ccf-mtDNA levels is associated with the activation of the cGAS-STING pathway, thereby triggering inflammation in MDs.






Similar content being viewed by others
References
Craven L, Alston C, Taylor R, Turnbull D (2017) Recent advances in mitochondrial disease. Annu Rev Genomics Hum Genet 18:257–275. https://doi.org/10.1146/annurev-genom-091416-035426
El-Hattab AW, Adesina AM, Jones J, Scaglia F (2015) MELAS syndrome: clinical manifestations, pathogenesis, and treatment options. Mol Genet Metab 116(1–2):4–12. https://doi.org/10.1016/j.ymgme.2015.06.004
Balsa E, Perry EA, Bennett CF et al (2020) Defective NADPH production in mitochondrial disease complex I causes inflammation and cell death. Nat Commun 11(1):2714. https://doi.org/10.1038/s41467-020-16423-1
Jin Z, Wei W, Yang M, Du Y, Wan Y (2014) Mitochondrial complex I activity suppresses inflammation and enhances bone resorption by shifting macrophage-osteoclast polarization. Cell Metab 20(3):483–498. https://doi.org/10.1016/j.cmet.2014.07.011
Soustek MS, Balsa E, Barrow JJ et al (2018) Inhibition of the ER stress IRE1alpha inflammatory pathway protects against cell death in mitochondrial complex I mutant cells. Cell Death Dis 9(6):658. https://doi.org/10.1038/s41419-018-0696-5
Perry EA, Bennett CF, Luo C et al (2021) Tetracyclines promote survival and fitness in mitochondrial disease models. Nat Metab 3(1):33–42. https://doi.org/10.1038/s42255-020-00334-y
Maresca A, Del Dotto V, Romagnoli M et al (2020) Expanding and validating the biomarkers for mitochondrial diseases. J Mol Med (Berl) 98(10):1467–1478. https://doi.org/10.1007/s00109-020-01967-y
Trifunov S, Paredes-Fuentes AJ, Badosa C et al (2021) Circulating cell-free mitochondrial DNA in cerebrospinal fluid as a biomarker for mitochondrial diseases. Clin Chem 67(8):1113–1121. https://doi.org/10.1093/clinchem/hvab091
Deng J, Lu Y, Xie Z, Liu J, Yuan Y, Wang Z (2020) RNA-seq profiling, and impaired autophagic process in skeletal muscle of MELAS. Biochem Biophys Res Commun 523(1):91–97. https://doi.org/10.1016/j.bbrc.2019.12.005
Borsche M, Konig IR, Delcambre S et al (2020) Mitochondrial damage-associated inflammation highlights biomarkers in PRKN/PINK1 parkinsonism. Brain 143(10):3041–3051. https://doi.org/10.1093/brain/awaa246
Gambardella S, Limanaqi F, Ferese R et al (2019) ccf-mtDNA as a potential link between the brain and immune system in neuro-immunological disorders. Front Immunol 10:1064. https://doi.org/10.3389/fimmu.2019.01064
Mehta SR, Perez-Santiago J, Hulgan T et al (2017) Cerebrospinal fluid cell-free mitochondrial DNA is associated with HIV replication, iron transport, and mild HIV-associated neurocognitive impairment. J Neuroinflamm 14(1):72. https://doi.org/10.1186/s12974-017-0848-z
Arshad O, Gadawska I, Sattha B, Cote HCF, Hsieh AYY; Canadian Institutes of Health Research Team on Cellular A, Women HIVCi, Children (2018) Elevated cell-free mitochondrial DNA in filtered plasma is associated with HIV infection and inflammation. J Acquir Immune Defic Syndr 78(1):111–118. https://doi.org/10.1097/QAI.0000000000001650
Barichello T, Savi GD, Simoes LR et al (2010) Evaluation of mitochondrial respiratory chain in the brain of rats after pneumococcal meningitis. Brain Res Bull 82(5–6):302–307. https://doi.org/10.1016/j.brainresbull.2010.05.012
Gopinathan U, Ovstebo R, Brusletto BS et al (2017) Transcriptomic data from two primary cell models stimulating human monocytes suggest inhibition of oxidative phosphorylation and mitochondrial function by N. meningitidis which is partially up-regulated by IL–10. BMC Immunol. https://doi.org/10.1186/s12865-017-0229-5
Blot M, Pauchard LA, Dunn I et al (2018) Mechanical ventilation and Streptococcus pneumoniae pneumonia alter mitochondrial homeostasis. Sci Rep 8(1):11718. https://doi.org/10.1038/s41598-018-30226-x
West AP, Khoury-Hanold W, Staron M et al (2015) Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520(7548):553–557. https://doi.org/10.1038/nature14156
White MJ, McArthur K, Metcalf D et al (2014) Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell 159(7):1549–1562. https://doi.org/10.1016/j.cell.2014.11.036
Sun L, Wu J, Du F, Chen X, Chen ZJ (2013) Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339(6121):786–791. https://doi.org/10.1126/science.1232458
Chen Q, Sun L, Chen ZJ (2016) Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol 17(10):1142–1149. https://doi.org/10.1038/ni.3558
Fang C, Wei X, Wei Y (2016) Mitochondrial DNA in the regulation of innate immune responses. Protein Cell 7(1):11–16. https://doi.org/10.1007/s13238-015-0222-9
Bai J, Cervantes C, Liu J et al (2017) DsbA-L prevents obesity-induced inflammation and insulin resistance by suppressing the mtDNA release-activated cGAS-cGAMP-STING pathway. Proc Natl Acad Sci USA 114(46):12196–12201. https://doi.org/10.1073/pnas.1708744114
Yu CH, Davidson S, Harapas CR et al (2020) TDP-43 triggers mitochondrial DNA release via mPTP to activate cGAS/STING in ALS. Cell 183(3):636-649 e18. https://doi.org/10.1016/j.cell.2020.09.020
Ling F, Zhao J, Liu J et al (2015) Clinical and muscle imaging findings in 14 mainland Chinese patients with oculopharyngodistal myopathy. PLoS ONE. https://doi.org/10.1371/journal.pone.0128629
Angela P, Brennan R, Marzena KA et al (2015) Reduced cerebrospinal fluid mitochondrial DNA Is a biomarker for early-stage Parkinson’s disease. Ann Neurol 78(6):1000–1004. https://doi.org/10.1002/ana.24515
Liu P, Li F, Lin J et al (2021) m(6)A-independent genome-wide METTL3 and METTL14 redistribution drives the senescence-associated secretory phenotype. Nat Cell Biol 23(4):355–365. https://doi.org/10.1038/s41556-021-00656-3
Kanehisa M, Araki M, Goto S et al (2008) KEGG for linking genomes to life and the environment. Nucleic Acids Res 36(Database issue):D480-484. https://doi.org/10.1093/nar/gkm882
Mancuso M, Orsucci D, Ienco EC et al (2013) An “inflammatory” mitochondrial myopathy a case report. Neuromuscul Disord 23(11):907–910. https://doi.org/10.1016/j.nmd.2013.07.011
West AP, Shadel GS (2017) Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat Rev Immunol 17(6):363–375. https://doi.org/10.1038/nri.2017.21
Zhang Q, Raoof M, Chen Y et al (2010) Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464(7285):104–107. https://doi.org/10.1038/nature08780
Zhang Q, Itagaki K, Hauser CJ (2010) Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock 34(1):55–59. https://doi.org/10.1097/SHK.0b013e3181cd8c08
Roers A, Hiller B, Hornung V (2016) Recognition of endogenous nucleic acids by the innate immune system. Immunity 44(4):739–754. https://doi.org/10.1016/j.immuni.2016.04.002
Barber GN (2015) STING: infection, inflammation and cancer. Nat Rev Immunol 15(12):760–770. https://doi.org/10.1038/nri3921
Lepelley A, Mina ED, Van Nieuwenhove E et al (2021) Enhanced cGAS-STING-dependent interferon signaling associated with mutations in ATAD3A. J Exp Med. https://doi.org/10.1084/jem.20201560
Li Q, Liu C, Yue R et al (2019) cGAS/STING/TBK1/IRF3 signaling pathway activates bmdcs maturation following Mycobacterium bovis infection. Int J Mol Sci. https://doi.org/10.3390/ijms20040895
Grabosch S, Bulatovic M, Zeng F et al (2019) Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene 38(13):2380–2393. https://doi.org/10.1038/s41388-018-0581-9
Zhao H, Wu L, Yan G et al (2021) Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther 6(1):263. https://doi.org/10.1038/s41392-021-00658-5
Tsai CH, Chen CJ, Gong CL et al (2021) CXCL13/CXCR5 axis facilitates endothelial progenitor cell homing and angiogenesis during rheumatoid arthritis progression. Cell Death Dis 12(9):846. https://doi.org/10.1038/s41419-021-04136-2
Denton AE, Innocentin S, Carr EJ et al (2019) Type I interferon induces CXCL13 to support ectopic germinal center formation. J Exp Med 216(3):621–637. https://doi.org/10.1084/jem.20181216
Koti M, Chenard S, Nersesian S, Vidotto T, Morales A, Siemens DR (2019) Investigating the STING pathway to explain mechanisms of BCG failures in non-muscle invasive bladder cancer: prognostic and therapeutic implications. Bladder Cancer 5(3):225–234. https://doi.org/10.3233/blc-190228
Sliter DA, Martinez J, Hao L et al (2018) Parkin and PINK1 mitigate STING-induced inflammation. Nature 561(7722):258–262. https://doi.org/10.1038/s41586-018-0448-9
Acknowledgements
We appreciated the cooperation of the patients and their families.
Funding
The work was funded by the National Natural Science Foundation of China (No. 81571219, 82071409, 82171846 and U20A20356). Capital's Funds for Health Improvement and Research (2022-4-40716).
Author information
Authors and Affiliations
Contributions
ZXW and JWD conceived the idea, designed studies and supervised the project; XTZ and MY designed and carried out experiments, analyzed data; MY, YWZ, YMZ, ZYX, and LCM collected the patient; QQW, JL, HL, WZ, YY contributed to the clinical diagnosis and biopsy of MD patients; XTZ, ZXW and JWD wrote and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, X., Yu, M., Zhao, Y. et al. Circulating cell-free mtDNA release is associated with the activation of cGAS-STING pathway and inflammation in mitochondrial diseases. J Neurol 269, 4985–4996 (2022). https://doi.org/10.1007/s00415-022-11146-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11146-3