Abstract
Mini-Mental State Examination (MMSE) lacks of sensitivity in detecting cognitive deficits associated with subcortical damage. The HIV-Dementia Scale (HDS), a screening tool originally created for detecting cognitive impairment due to subcortical damage in HIV + patients, has proved to be useful in other neurological diseases. Until now, an Italian version of the HDS is not available. We aimed at: (1) validating the HDS Italian version (HDS-IT) in a cohort of cognitively healthy subjects (CN); (2) exploring the suitability of HDS-IT in detecting cognitive impairment due to subcortical damage (scCI). The psychometric properties of the HDS-IT were assessed in 180 CN (mean age 67.6 ± 8.3, range 41–84) with regard to item-total correlation, test–retest reliability and convergent validity with MMSE. Item-total correlations ranged 0.44–0.72. Test–retest reliability was 0.70 (p < 0.001). The HDS-IT scores were positively associated with MMSE score (rS = 0.49, p < 0.001). Then, both the HDS-IT and the MMSE were administered to 44 scCI subjects (mean age 64.9 ± 10.6, range 41–84). Mean HDS-IT total score was close to the original version and significantly lower in the scCI group compared to CN (8.6 ± 3.6 vs. 12.6 ± 2.5, p < 0.001). ROC analysis yielded an optimal cutoff value of 11, with sensitivity of 0.70 and specificity of 0.82. Patients showed poorer scores on HDS-IT compared to CN (12.6 ± 2.5 vs. 8.6 ± 3.6, p < 0.001). Our results support the use of HDS-IT as a screening tool suitable for detecting cognitive deficits with prevalent subcortical pattern, being complementary to MMSE in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early detection of cognitive impairment in the ageing population represents an important issue. It is known that mild cognitive impairment (MCI) in adult patients is a frequent and heterogeneous condition that may be related to different underlying causes, especially neurodegenerative or cerebrovascular diseases [1, 2]. Neurological disorders affecting the central nervous system are associated with a wide spectrum of clinical manifestations, often accompanied by presence of such cognitive dysfunctions, and in some cases, dementia. To optimise the diagnostic workup, subjects should undergo a screening assessment for the characterisation of global cognitive profile, followed by an extensive assessment if cognitive deficits are detected [3]. Accordingly, an ideal screening tool should be relatively simple to administered, not time consuming and sensitive enough to allow the identification of patients deserving further, in-depth neuropsychological assessment [4]. In particular, screening tests for the assessment of global cognitive functioning should be able to highlight cognitive profiles with prevalent cortical (i.e. deficits in declarative memory, language, praxis and visuospatial abilities) vs. subcortical (i.e. deficits in attention and arousal, memory retrieval, speed of information processing, motivation and mood) pattern of cognitive impairment, so that clinicians may be better oriented in their examination. [5,6,7]. Anatomically, the cortical pattern is related to diseases involving primarily, but not exclusively, the association cortex of the cerebral hemispheres and the medial temporal lobes, and are typically characterised by aphasia, amnesia, agnosia, acalculia, and apraxia. The subcortical pattern occurs in disorders with predominant involvement of basal ganglia, thalamus, and structures of the brainstem, and is typically characterised by psychomotor slowing, memory impairment, affective and emotional disorders, and difficulties with strategy formation and problem solving [8]. However, though the historic cortical vs. subcortical dichotomy may be useful to identify preeminent neuropsychological profiles in clinical practice [9], the existence of “true” cortical and subcortical disorders is controversial from a functional/neuroanatomical perspective [10].
In clinical practice, the most popular screening tool for assessing global functioning is the Mini-Mental State Examination (MMSE) [11]. MMSE is composed by several items, most of them requiring the integrity of higher cortical functions (memory, language, orientation and visuo-constructive praxis). However, MMSE lacks of items assessing executive functions; so far, its sensitivity in detecting subcortical patterns of cognitive impairment is low [7, 12,13,14]. Several studies reported that the Montreal Cognitive Assessment (MoCA), another well-known screening test, is superior to MMSE for the early detection of cognitive impairment in ageing population [14, 15], due to its item composition. In fact, the MoCA is more suitable than MMSE in assessing visuospatial and executive functions, representing a more challenging task to be used in clinical practice [3]. On the other hand, a brief bedside screening tool as the Frontal Assessment Battery (FAB) is ideal for the assessment of executive functions, despite, it cannot replace measures of global cognition as the MMSE. Studies that investigated the utility of FAB for differential diagnosis among different dementias gave mixed results, showing more suitability of specific FAB sub-items than the FAB total score in distinguishing cortical dementias, as Alzheimer’s disease and fronto-temporal lobar dementia, and subcortical vascular cognitive impairment [16,17,18]. However, none of the abovementioned screening tools is adequate for assessing reaction times and speed processing, because both of them lack of time-dependent items.
Actually, subcortical cognitive impairment (scCI) is mainly related to damage in specific subcortical brain regions (i.e. thalamus, basal ganglia, midbrain), but it may also be a consequence of disruption in white matter connection fibres (white matter lesions, WMLs). Accordingly, pictures of WMLs, that may disrupt cortico-cortical intra- and inter-hemispheric, as well as cortico-subcortical connections, may cause this kind of cognitive impairment [19]. scCI represents a clinical feature of many neurological diseases, such as subcortical ischaemic vascular disease (SIVD), normal pressure hydrocephalus (NPH), multiple sclerosis (MS), Huntington’s disease (HD), Parkinson’s disease (PD), and progressive supranuclear palsy (PSP) [20]. Nowadays, it is known that, in clinical practice, an accurate neuropsychological assessment may allow to early detect clinical manifestations of these neurological disease prior to the dementia phase. Among the available tools for scCI detection, none is suitable to be applied as screening measure in clinical practice [21]. So far, a sensitive tool for revealing features of subcortical cognitive impairment is strongly recommended. The HIV-Dementia Scale (HDS) is a brief tool originally developed to assess subcortical deficits in individuals affected by HIV infection [22]. Since HDS proved to be useful in detecting scCI in HIV + patients, its suitability for detecting cognitive impairment in other neurological diseases with subcortical damage has been assessed, giving significant results in NPH and SIVD [21].
Later on, the International version of the HIV-Dementia Scale (I-HDS) [12] has been validated as a cross-cultural screening test to use for detection of HIV dementia within the worldwide community. Until now, normative data for the Italian population are lacking.
The objectives of this study are: (1) to carry out the validation of the HDS Italian version in a cohort of cognitively healthy elderly subjects (CN), and (2) to explore its sensitivity and specificity in detecting subcortical cognitive deficits in a clinical sample of subjects with neurological diseases associated with subcortical damage (scCI).
Materials and methods
Participants and assessment procedure
We enrolled 180 CN recruited among relatives of patients attending our Memory Clinic or as volunteers after advertisement. The participants’ inclusion criteria included: (i) age between 40 and 85, (ii) good physical and mental health, (iii) no concomitant uncontrolled medical diseases, (iv) Mini-Mental State Examination raw score ≥ 24, and (v) no dementia. Patients were classified as CN by means of extensive neuropsychological evaluation (see section below) assessing multiple cognitive domains. Scores within normal range in all cognitive domains led us to define a patient as CN. A subgroup of 27 subjects repeated the HDS-IT after a mean test–retest interval of 3–10 months (median 7).
We also enrolled 44 consecutive patients attending to our Memory Clinic for neurological disorders with subcortical features: 13 with multiple sclerosis (MS), 16 with subcortical ischaemic vascular disease (SIVD), 9 with normal pressure hydrocephalus (NPH) and 6 with HIV+ infection (HIV+). For patients with diagnosis of MS, we adopted the radiological criteria of minimum 4–9 white matter lesions [23]. For SIVD patients, we adopted the radiological criteria of score 2–3 in the Fazekas scale [24]. Patients with NPH were included on the basis of clinico-radiological diagnosis. Patients with HIV+ were included on the basis of serological diagnosis.
All of them showed subcortical cognitive impairment (scCI). ScCI was defined as a score ≥ 1.5 SD below the adjusted-mean in one or more cognitive domains, evaluated through an extensive neuropsychological battery, in patients with neurological disorders associated with subcortical damage. A clinical condition of dementia was excluded for all patients.
Neuropsychological testing
All subjects underwent the following neuropsychological battery: the MMSE [25] for the assessment of global cognitive functioning; the Rey Auditory Verbal Learning Test [26] for the evaluation of verbal learning and memory; the digit span forward and backward [27] for the evaluation of verbal short-term memory and working memory; the Trail Making Test-part A and B [28] for the evaluation of visuospatial selective and divided attention and mental shifting; the copy of drawings and copy of drawings with landmarks [26] and the Clock Drawing Test [29] for the evaluation of visuo-constructive praxis; the Raven’s coloured progressive matrices ‘47 [30] for the evaluation of abstract logical reasoning; the phonemic fluency [31] and category fluency [32] for the evaluation of language. Clinical staging was assessed by means of the Clinical Dementia Rating Scale (CDR) [33].
The original version of HIV-Dementia Scale
The HDS original version consists of four subtests. Item 1—attention (max score = 4): modified from anti-saccadic error task [34]. The patient is asked to look the examiner’s nose and then to focus on examiner’s moving index finger, repeating the task with alternating hands. When the patient is comfortable, looking at the finger that moves, the examiner ask him/her to look at the not moving index finger. This task is practised until the patient becomes familiar with the procedure. Then the patient is asked to perform 20 serial anti-saccades. An error is marked when the patient looks towards the moving finger. Item 2—psychomotor speed (max score = 6): patient is asked to write the entire alphabet. If the patient is unable to perform it correctly, the examiner asks him/her to write the numbers from 1 to 26 and the time taken is recorded. The time taken to complete this task is converted into a numerical value from 0 to 6. Items 3—memory recall (max score = 4): the patient is asked to repeat and remember four words. The four words to be recorded in the HDS-IT memory subtest correspond to the translation of the original version (“dog”, “hat”, “green” and “peach”) [21]. Item 4—construction speed (max score = 2): the patient is asked to the draw of a copy cube. Primarily the examiner explains the figure copy, the time needed to copy is recorded and converted into a numerical score from 0 to 2. The maximum HDS score is 16.
Development of the Italian version
The HDS-IT was developed using forward–backward translation. Two researchers separately translated the English version into Italian, and then compared the two translations. The resulting draft was translated back into English by a native independent English speaker fluent in Italian language who did not know the original version of the scale. The Italian version was compared with the original English version, any discrepancy was discussed and a final version was adopted after reaching the full agreement. This final Italian version is reported in Supplementary file. As with the original English version, in HDS-IT the item of psychomotor speed consists of writing the numbers from 1 to 21 (instead of 1–26 as in the original version), due to Italian alphabet, composed of 21 letters rather than 26 as the English one.
As for the original version, the maximum score is 16.
To avoid interference with the recall of words (for instance, ‘CAPPELLO’ may interfere with the RAVLT list), we suggest that the administration of the test should be done at least 15 min before the verbal memory test.
Statistical analysis
The data were analysed using R version 3.5. Descriptive statistics were calculated. Student T test was applied to test significance of differences of continuous variables between scCI and CN. Mann–Whitney U test was used whenever appropriate. Gender difference between the groups was assessed via Chi-Square Test. Test–retest variability was calculated with Spearman correlation coefficient. Receiver-operating characteristics (ROC) curve analysis was carried out for evaluating accuracy of HDS in discriminating scCI from controls. The optimal cutoff value was determined according to Youden Index. AUC, sensitivity and specificity were provided along with their 95% CI according with the selected cutoff. In all the analyses, two-sided p values < 0.05 were considered as statistically significant.
Results
Demographic characteristics of participants are reported in Table 1. CN and scCI did not differ in the distribution of age, gender and education. CN subjects showed lower MMSE mean scores compared to scCI (26.2 ± 2.8 vs. 28.3 ± 1.3, p < 0.001).
Validation of the HDS Italian version
The HDS-IT total score was negatively associated with age (rS = − 0.18, p = 0.008), while it was positively associated with education (rS = 0.39, p < 0.001). No associations were found with gender (p = 0.571). HDS-IT and MMSE total scores were positively associated (rS = 0.49, p < 0.001).
Corrected item-total correlations ranged between 0.44 and 0.72. Moreover, the average of inter-item correlation was higher than 0.17. Test–retest reliability was assessed in 27 subjects, yielding a score of rS = 0.70 (p < 0.001). The mean duration between visits was 3–10 months (median: 7).
Exploring HDS-IT suitability in detecting subcortical cognitive deficits
Mean HDS-IT total score was close to the original version in both groups and significantly lower in the scCI compared to CN group (12.6 ± 2.5 vs. 8.6 ± 3.6, p < 0.001). Performing a sub-analysis comparing single items in the two groups, we found significant differences for item 2 (5.2 ± 1.4 vs. 2.7 ± 2.5, p < 0.001), and a trend toward significance for item 3 (3.0 ± 0.9 vs. 2.1 ± 1.2, p = 0.004). All complete results are shown in Table 2.
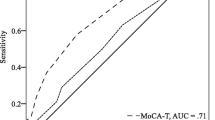
To determine the optimal cutoff score for identifying scCI versus CN with the HDS-IT, a ROC curve was constructed (Fig. 1) and was adjusted for age. The ROC curve (AUC = 0.80, 95% CI 0.73–0.88) yielded an optimal cutoff value for an HDS-IT score of ≤ 11. Based on this cutoff value, the sensitivity was 0.70 (95% CI 0.48–0.84) and the specificity 0.82 (95% CI 0.65–0.97). A score of ≤ 11 was also able to discriminate those scCI with MMSE ≥ 24 (37/44) vs. CN, with a sensitivity of 0.65 (95% CI 0.49–0.81) and a specificity of 0.79 (95% CI 0.73–0.85).
Discussion
The purposes of this study were to validate the Italian version of the HDS (HDS-IT) in a cohort of cognitively healthy elderly subjects and to explore its suitability as a sensitive screening tool for detecting subcortical cognitive impairment in subjects with neurological diseases associated with subcortical damage (scCI).
With respect to the first aim (validation of the HDS-IT in a cohort of cognitively healthy volunteers), our results displayed that the HDS-IT revealed good psychometric properties as well as the original version, shown by the criterion validity and test–retest reliability. Regarding the criterion validity, we found a trend in convergent validity between HDS and MMSE (i.e. the better the MMSE score, the better the HDS score). However, these measures did not overlap, because of their complementarity due to different items composition. About test–retest reliability, we found a robust test–retest correlation (rS = 0.70) with a mean interval of 3–10 months. Our result was in line with a previous study that found good performance at 3–9 weeks interval [22] and at 4 months [35]. The use of a wider time interval in our study may represent an advantage to control for possible learning or practice effect, defined as “the capability of an individual to learn and adjust” after repeated administration of a task [36]. This represents a critical issue in clinical practice, since this can affect the test–retest reliability of a task, particularly in cognitively healthy subjects [37, 38]. Furthermore, we found that the HDS-IT was inversely associated with age and positively associated with education, whereas it was independent from gender. Our findings are in line with that observed for other screening tests, so a correction for age and education should be considered in future studies, for a better interpretation of the raw scores obtained at the HDS-IT.
With regard to the second aim (i.e. the behaviour of the HDS-IT in a clinical sample of scCI patients compared to healthy control), we found that patients with scCI showed poorer scores on the HDS-IT compared to cognitively healthy individuals, even in those with MMSE < 28. This observation further supports the sensitivity of the HDS-IT in detecting cognitive deficits with prevalent subcortical pattern.In particular, those scCI patients who displayed normal scores on MMSE frequently displayed low scores on the HDS-IT. A previous study found significant correlations of the HDS scores with neuropsychological measures exploring attention/working memory, processing speed and executive functions, supporting the usefulness of this test for detection of subcortical cognitive deficits [39]. In our study, the capability of HDS-IT in detecting subcortical cognitive impairment was accomplished by comparing HDS-IT performance between patients with scCI and CN. Overall, our results support the use of HDS as a screening tool for detecting subcortical cognitive deficits, being complementary to MMSE in clinical practice.
Our study has some limitations. While all patients in the scCI group underwent a neuroimaging acquisition, this was not provided for some individuals in the control group. Therefore, the actual vascular load (i.e. white matter hyperintensities and subcortical damage) might have been underestimated in the control group. Moreover, the scCI group was heterogeneous in terms of diagnosis, although all of the patients shared a subcortical pathophysiology underlying their neurological disease. Only 6 patients with HIV infection have been included, diagnosed on the basis of clinical and laboratory data, while no brain imaging data were available. Therefore, in the present study, the suitability of the Italian version of the HDS in the original test’s target population has not been replicated.
Further studies have to be performed to validate the HDS in other diseases causing scCI, such as Parkinson’s disease or Huntington’s disease, as recommended previously [21]. Furthermore, future studies can be useful to explore the performance at HDS-IT also in cortical-type neurodegenerative diseases (i.e. Alzheimer’s disease), where a subcortical damage may be involved in the pathogenesis and accompany the neurodegenerative processes, though at a lower level of magnitude.
In conclusion, our results suggest that the HDS-IT is able to detect subcortical deficits in a population of patients with subcortical neurological disorders (i.e. SIVD, NPH and MS and HIV+). The HDS-IT showed good psychometric properties, so it may represent a suitable screening tool to be used in clinical practice, being complementary to MMSE.
Data availability
Data and material are available upon reasonable request.
References
Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256(3):183–194. https://doi.org/10.1111/j.1365-2796.2004.01388.x
Gillis C, Mirzaei F, Potashman M, Ikram MA, Maserejian N (2019) The incidence of mild cognitive impairment: a systematic review and data synthesis. Alzheimers Dement (Amst) 11:248–256. https://doi.org/10.1016/j.dadm.2019.01.004
Roalf DR, Moberg PJ, Xie SX, Wolk DA, Moelter ST, Arnold SE (2013) Comparative accuracies of two common screening instruments for classification of Alzheimer’s disease, mild cognitive impairment, and healthy aging. Alzheimers Dement 9(5):529–537. https://doi.org/10.1016/j.jalz.2012.10.001
de Almeida SM, Kamat R, Cherner M, Umlauf A, Ribeiro CE, de Pereira AP, Franklin D, Heaton RK, Ellis RJ (2017) Improving detection of HIV-associated cognitive impairment: comparison of the international HIV dementia scale and a brief screening battery. J Acquir Immune Defic Syndr 74(3):332–338. https://doi.org/10.1097/QAI.0000000000001224 (Erratum in: J Acquir Immune Defic Syndr, 2017;76(2):e64)
Lezak MD, Howieson DB, Loring DW (2004) Neuropsychological assessment (4th edn). Oxford University Press, New York. https://doi.org/10.1007/s00415-007-2004-7
Shaik SS, Varma AR (2012) Differentiating the dementias: a neurological approach. Prog Neurol Psychiatry. https://doi.org/10.1002/pnp.224
Milanini B, Ciccarelli N, Fabbiani M, Baldonero E, Limiti S, Gagliardini R, Borghetti A, D’Avino A, Mondi A, Colafigli M, Cauda R, Di Giambenedetto S (2016) Neuropsychological screening tools in Italian HIV+ patients: a comparison of Montreal Cognitive Assessment (MoCA) and Mini Mental State Examination (MMSE). Clin Neuropsychol 30(sup1):1457–1468. https://doi.org/10.1080/13854046.2016.1183048
Cummings JL (1986) Subcortical dementia Neuropsychology, neuropsychiatry, and pathophysiology. Br J Psychiatry 149:682–697. https://doi.org/10.1192/bjp.149.6.682
Arango-Lasprilla JC, Rogers H, Lengenfelder J, Deluca J, Moreno S, Lopera F (2006) Cortical and subcortical diseases: do true neuropsychological differences exist? Arch Clin Neuropsychol 21(1):29–40. https://doi.org/10.1016/j.acn.2005.07.004
Salmon DP, Filoteo JV (2007) Neuropsychology of cortical versus subcortical dementia syndromes. Semin Neurol 27(1):7–21. https://doi.org/10.1055/s-2006-956751
Folstein MF, Folstein SE, McHugh PR (1975) ”Mini-mental state” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198. https://doi.org/10.1016/0022-3956(75)90026-6
Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, Robertson K, McArthur JC, Ronald A, Katabira E (2005) The International HIV Dementia Scale: a new rapid screening test for HIV dementia. AIDS 19(13):1367–1374. https://doi.org/10.1097/01.aids.0000194798.66670.6e
Trzepacz PT, Hochstetler H, Wang S, Walker B, Saykin AJ, Alzheimer’s Disease Neuroimaging Initiative. (2015) Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for assessment of mild cognitive impairment in older adults. BMC Geriatr 15:107. https://doi.org/10.1186/s12877-015-0103-3
Siqueira GSA, Hagemann PMS, Coelho DS, Santos FHD, Bertolucci PHF (2019) Can MoCA and MMSE be interchangeable cognitive screening tools? Syst Rev Gerontol 59(6):e743–e763. https://doi.org/10.1093/geront/gny126
Markwick A, Zamboni G, de Jager CA (2012) Profiles of cognitive subtest impairment in the Montreal Cognitive Assessment (MoCA) in a research cohort with normal Mini-Mental State Examination (MMSE) scores. J Clin Exp Neuropsychol 34(7):750–757. https://doi.org/10.1080/13803395.2012.672966
Lipton AM, Ohman KA, Womack KB, Hynan LS, Ninman ET, Lacritz LH (2005) Subscores of the FAB differentiate frontotemporal lobar degeneration from AD. Neurology 65(5):726–731. https://doi.org/10.1212/01.wnl.0000174437.73416.7b
Oguro H, Yamaguchi S, Abe S, Ishida Y, Bokura H, Kobayashi S (2006) Differentiating Alzheimer’s disease from subcortical vascular dementia with the FAB test. J Neurol 253(11):1490–1494. https://doi.org/10.1007/s00415-006-0251-7
Boban M, Malojcić B, Mimica N, Vuković S, Zrilić I (2012) The frontal assessment battery in the differential diagnosis of dementia. J Geriatr Psychiatry Neurol 25(4):201–207. https://doi.org/10.1177/0891988712464821
Mori E (2002) Impact of subcortical ischemic lesions on behavior and cognition. Ann NY Acad Sci 977:141–148. https://doi.org/10.1111/j.1749-6632.2002.tb04809.x
Filley CM (2019) History of subcortical cognitive impairment. Front Neurol Neurosci 44:108–117. https://doi.org/10.1159/000494958
van Harten B, Courant MN, Scheltens P, Weinstein HC (2004) Validation of the HIV Dementia Scale in an elderly cohort of patients with subcortical cognitive impairment caused by subcortical ischaemic vascular disease or a normal pressure hydrocephalus. Dement Geriatr Cogn Disord 18(1):109–114. https://doi.org/10.1159/000077818
Power C, Selnes OA, Grim JA, McArthur JC (1995) HIV Dementia Scale: a rapid screening test. J Acquir Immune Defic Syndr Hum Retrovirol 8(3):273–278. https://doi.org/10.1097/00042560-199503010-00008
Barkhof F, Filippi M, Miller DH, Scheltens P, Campi A, Polman CH, Comi G, Adèr HJ, Losseff N, Valk J (1997) Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 120(Pt11):2059–2069. https://doi.org/10.1093/brain/120.11.2059
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am J Roentgenol 149(2):351–356. https://doi.org/10.2214/ajr.149.2.351
Magni E, Binetti G, Bianchetti A, Rozzini R, Trabucchi M (1996) Mini-Mental State Examination: a normative study in Italian elderly population. Eur J Neurol 3(3):198–202. https://doi.org/10.1111/j.1468-1331.1996.tb00423.x
Carlesimo G, Caltagirone C, Gainotti G, Fadda L, Gallassi R, Lorusso S, Marfia G, Marra C, Parnetti L (1996) The mental deterioration battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The group for the standardization of the mental deterioration battery. Eur Neurol 36(6):378–384. https://doi.org/10.1159/000117297
Monaco M, Costa A, Caltagirone C, Carlesimo GA (2013) Forward and backward span for verbal and visuo-spatial data: standardization and normative data from an Italian adult population. Neurol Sci 34(5):749–754. https://doi.org/10.1007/s10072-012-1130-x (Epub 2012 Jun 12. Erratum in: Neurol Sci. 2015 Feb;36(2):345-347)
Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E (1996) Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci 17(4):305–309. https://doi.org/10.1007/BF01997792
Caffarra P, Gardini S, Zonato F, Concari L, Dieci F, Copelli S, Freedman M, Stracciari A, Venneri A (2011) Italian norms for the Freedman version of the Clock Drawing Test. J Clin Exp Neuropsychol 33(9):982–988. https://doi.org/10.1080/13803395.2011.589373
Basso A, Capitani E, Laiacona M (1987) Raven’s coloured progressive matrices: normative values on 305 adult normal controls. Funct Neurol 2:189–194
Novelli G, Papagno C, Capitani E, Laiacona M, Cappa SF, Vallar G (1986) Three clinical tests to research and rate the lexical performance of normal subjects. Arch Psicol Neurol Psichiatr 47:278–296
Spinnler H, Tognoni G (1987) Standardizzazione e taratura italiana di test neuropsicologici. Ital J Neurol Sci 6:21–120
Morris JC (1997) Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr 9(Suppl 1):173–176. https://doi.org/10.1017/s1041610297004870
Currie J, Ramsden B, McArthur C, Maruff P (1991) Validation of a clinical antisaccadic eye movement test in the assessment of dementia. Arch Neurol 48(6):644–648. https://doi.org/10.1001/archneur.1991.00530180102024
Lu GM, Brew BJ, Siefried KJ, Draper B, Cysique LA (2013) Is the HIV Dementia Scale a reliable tool for assessing HIV-related neurocognitive decline? J AIDS Clin Res 5:269. https://doi.org/10.4172/2155-6113.1000269
Bartels C, Wegrzyn M, Wiedl A, Ackermann V, Ehrenreich H (2010) Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC Neurosci 11:118. https://doi.org/10.1186/1471-2202-
Calamia M, Markon K, Tranel D (2012) Scoring higher the second time around: meta-analyses of practice effects in neuropsychological assessment. Clin Neuropsychol 26(4):543–570. https://doi.org/10.1080/13854046.2012.680913
Abner EL, Dennis BC, Mathews MJ, Mendiondo MS, Caban-Holt A, Kryscio RJ, Schmitt FA, PREADViSE Investigators, Crowley JJ; SELECT Investigators. (2012) Practice effects in a longitudinal, multi-center Alzheimer’s disease prevention clinical trial. Trials 13:217. https://doi.org/10.1186/1745-6215-13-217.11-118
Marin-Webb V, Jessen H, Kopp U, Jessen AB, Hahn K (2016) Validation of the International HIV Dementia Scale as a screening tool for HIV-associated neurocognitive disorders in a German-speaking HIV outpatient clinic. PLoS ONE 11(12):e0168225. https://doi.org/10.1371/journal.pone.0168225
Funding
Open access funding provided by Università degli Studi di Perugia within the CRUI-CARE Agreement. The present study had no external funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest.
Ethics approval
The study protocol was approved by the Local Ethics Committee (CEAS Umbria).
Consent to participate
All participants gave their written consent.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Montanucci, C., Chipi, E., Salvadori, N. et al. HIV-Dementia Scale as a screening tool for the detection of subcortical cognitive deficits: validation of the Italian version. J Neurol 268, 4789–4795 (2021). https://doi.org/10.1007/s00415-021-10592-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10592-9