Abstract
Background
The presence of metabolically viable brain tissue that may be salvageable with rapid cerebral blood flow restoration is the fundament rationale for reperfusion therapy in patients with large vessel occlusion stroke. The effect of endovascular treatment (EVT) on functional outcome largely depends on the degree of recanalization. However, the relationship of recanalization degree and penumbra salvage has not yet been investigated. We hypothesized that penumbra salvage volume mediates the effect of thrombectomy on functional outcome.
Methods
99 acute anterior circulation stroke patients who received multimodal CT and underwent thrombectomy with resulting partial to complete reperfusion (modified thrombolysis in cerebral infarction scale (mTICI) ≥ 2a) were retrospectively analyzed. Penumbra volume was quantified on CT perfusion and penumbra salvage volume (PSV) was calculated as difference of penumbra and net infarct growth from admission to follow-up imaging.
Results
In patients with complete reperfusion (mTICI ≥ 2c), the median PSV was significantly higher than the median PSV in patients with partial or incomplete (mTICI 2a–2b) reperfusion (median 224 mL, IQR: 168–303 versus 158 mL, IQR: 129–225; p < 0.01). A higher degree of recanalization was associated with increased PSV (+ 63 mL per grade, 95% CI: 17–110; p < 0.01). Higher PSV was also associated with improved functional outcome (OR/mRS shift: 0.89; 95% CI: 0.85–0.95, p < 0.0001).
Conclusions
PSV may be an important mediator between functional outcome and recanalization degree in EVT patients and could serve as a more accurate instrument to compare treatment effects than infarct volumes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mechanical thrombectomy (MT) in acute ischemic stroke substantially improves functional outcome in patients with large vessel occlusion [18, 34]. Yet, the time-sensitive selection of patients who will most likely benefit from MT is a critical factor in clinical practice. Neuroimaging may be used to guide endovascular treatment, and may serve as a prognostic biomarker [1, 2, 35]. Past MT landmark trials including patients 0–6 h from symptom onset applied different brain imaging criteria for treatment selection, for instance using computed tomography (CT) perfusion to estimate ischemic core volume (i.e. volume that is thought to represent irreversible tissue injury), compared to the total volume of hypoperfused brain tissue [1, 22, 28]. Accordingly, the presence of ischemic penumbra (metabolically viable brain tissue that may be salvageable with rapid cerebral blood flow restoration) is the fundamental rationale for reperfusion therapy [11]. However, the effect of endovascular treatment on functional outcome highly depends on the degree of recanalization as exemplified in previous studies [15, 20, 21]. Recently, a meta-analysis found an incremental association between the degree of recanalization and clinical outcome [21]. Currently, the American Heart Association (AHA) guidelines recommend a score of ≥ 2b on the modified Thrombolysis in Cerebral Infarction (mTICI) scale as the angiographic goal of MT [29, 30]. However, a wide range of outcome is still evident even in cases of successful reperfusion, indicating that outcome is completely mediated by further baseline and procedural covariates [9, 21].
Currently, it remains uncertain how the volume of penumbra salvage (PSV) mediates the effect of thrombectomy on functional outcome. Moreover, the relationship of penumbra salvage and the degree of recanalization has not yet been investigated.
We hypothesized twofold: First, a higher degree of recanalization is incrementally associated with higher PSV. Second, we hypothesized that PSV is directly linked to functional outcome.
Materials and methods
Patients
For this retrospective study, we consecutively analyzed all ischemic stroke patients with acute large vessel occlusion of the middle cerebral artery admitted between June 2015 and October 2019 at our university hospital, which is a high-volume tertiary stroke center (> 300 stroke thrombectomy procedures per year). Only anonymized data were analyzed after ethical review board approval, and the local ethics committee (Ethikkommission der Ärztekammer Hamburg) waived informed consent after review. The data that support the findings of this study are available from the corresponding author upon reasonable request. The study was conducted in accordance with the ethical guidelines (“Leitlinien der Ärztekammer Hamburg”) of the local ethics committee and in accordance with the Declaration of Helsinki.
All ischemic stroke patients admitted in the aforementioned time period were screened based on the following a priori defined inclusion criteria: (1) acute anterior circulation stroke in the territory of the middle cerebral artery (MCA) and MCA occlusion; (2) multimodal CT imaging protocol at admission including CT Angiography (CTA) and CT Perfusion (CTP); (3) known time window from symptom onset to admission imaging; (4) follow-up CT (FCT) 24 h after admission (max. range 23–25 h after onset); (5) admission National Institutes of Health Stroke Scale (NIHSS) score above 3; (6) documented functional outcome after 3 months based on modified Ranking Scale (mRS) score; (7) Absence of intracranial hemorrhage with significant mass effect (parenchymal hemorrhage (PH) type 2) according to Fiorelli et al. [14] and preexisting thromboembolic or hemodynamic infarctions in admission non-enhanced CT (NECT) or preexisting significant carotid stenosis; (8) Absence of significant motion artifacts.
Only patients fulfilling all criteria were included in this study. Baseline patient characteristics were retrieved from the medical records, including the modified Rankin Scale (mRS) score after 90 days.
Recanalization rates were classified as complete, incomplete and partial recanalization by the responsible neuroradiologists according to the mTICI score; reperfusion grade 2a indicates antegrade reperfusion of less than half of the occluded target artery previously ischemic territory; grade 2b, antegrade reperfusion of more than half of the previously occluded target artery ischemic territory; grade 2c, near-complete perfusion except for slow flow in a few distal cortical vessels or presence of small distal cortical emboli and grade 3, complete antegrade reperfusion of the previously occluded target artery ischemic territory, with absence of visualized occlusion in all distal branches.
Complete recanalization was defined as mTICI 2c/3, based on recent studies recommending this recanalization degree as primary aim of MT [12]. Patients with complete recanalization were compared to patients with successful but incomplete (mTICI 2b), and partial recanalization (mTICI 2a). For dichotomized analysis, patients with complete recanalization were compared to patients with mTICI 2a–2b.
A binary clinical outcome was defined based on modified Rankin Scale (mRS) after 90 days with 0–2 as functional independence and mRS ≥ 3 as poor outcome.
Image acquisitions
All patients received multimodal stroke imaging at admission with NECT, CTA, and CTP performed in equal order on 256 dual slice scanners (Philips iCT 256). NECT: 120 kV, 280–340 mA, 5.0 mm slice reconstruction, 1 mm increment; CTA: 100–120 kV, 260–300 mAs, 5.0-mm slice reconstruction, 1-mm increment, 80 mL highly iodinated contrast medium and 50 mL NaCl flush at 4 mL/s; CTP: 80 kV, 200–250 mA, 5 mm slice reconstruction (max. 10 mm), slice sampling rate 1.50 s (min. 1.33 s), scan time 45 s (max. 60 s), biphasic injection with 30 ml (max. 40 ml) of highly iodinated contrast medium with 350 mg iodine/ml (max. 400 mg/ml) injected with 6 ml/s, followed by a 30 ml sodium chloride chaser bolus.
CT-perfusion analysis
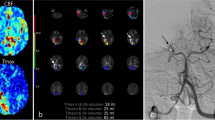
Infarct core and penumbra have been assessed using CT-perfusion (CTP) with whole brain coverage. Penumbra has been determined using relative mean transit time (MTT) with a threshold of 145% and infarct core has been defined using absolute cerebral blood volume (CBV) with a threshold at 2.0 ml × 100 g−1, as described by Wintermark et al. [36]. Based on the CTP-derived volumes for ischemic core and hypoperfusion volumes, we calculated penumbra volumes as their difference (Eq. 1). Secondly, we determined net infarct growth from admission CT to FCT based on the difference of the total infarct volume in FCT and ischemic core in admission CT (Eq. 2). Finally, we subtracted the net infarct growth volume from penumbra volume to determine penumbra salvage volume (PSV) (Eq. 3). Figure 1 illustrates a case, and how PSV was determined.
Quantification of penumbra salvage volume. Illustration of the quantification of penumbra salvage volume (PSV). Baseline non-enhanced CT is displayed on the left hand side (a), and perfusion imaging besides (b for ischemic core, c for hypoperfusion volume). Follow-up CT is displayed on the right hand side, where follow-up infarct volume was calculated
Anonymized data was processed at an imaging core lab. Image analysis including volumetric analysis was performed using commercially available software (Analyze 11.0, Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). All analyses were conducted by an experienced neuroradiologist (> 10 years of experience). Subsequently, all cases were screened in a consensus reading with a second experienced neuroradiologist.
Statistical analysis
Univariable distribution of metric variables is described by median and interquartile range (IQR). Absolute and relative frequencies are given for categorical data. To compare two independent samples regarding a metric or categorical outcome we used Mann–Whitney U test or χ2 test, respectively (Table 1). The impact of recanalization degree on PSV was illustrated in boxplots (Fig. 2).
Univariable and multivariable linear regression analyses were performed with PSV as dependent variable, and age, ASPECTS, core lesion volume, penumbra volume, application of intravenous alteplase, degree of recanalization (partial, incomplete, complete), NIHSS, and time from onset to recanalization as independent parameters. For multivariable analysis, backward selection was used integrating all above-mentioned variables that showed a significant association to PSV in univariable analysis (Table 2). The impact of recanalization degree on PSV according to the baseline penumbra volume is shown in Fig. 3.
Secondly, uni- and multivariable ordinal regression analyses were performed with modified Ranking Scale score at day 90 (mRS) as dependent variable using the same aforementioned independent variables. The ordinal form of the day 90 mRS was chosen due to its better relation to long-term outcomes in patients following ischemic stroke than dichotomized mRS [16] (Table 2). Figure 4 shows effect plots for ordinal regression analysis with probability for mRS shift (y-axis) depending on baseline ischemic core volume (x-axis) separately for different levels of PSV. Further effect plots are displayed in the supplemental material (Supplemental Fig. 1 for ASPECTS, Supplemental Fig. 2 for age).
Finally, two multivariate logistic regression models to predict functional independence were tested against each other to determine the additional value of PSV for the prediction of functional outcome. Both models included baseline ischemic core volume, penumbra volume, adjusted for age and degree of recanalization. In model 2, PSV was added as further variable. For both models (model 1 − PSV; model 2 + PSV), the predictive values were plotted against each other using receiver operating characteristic (ROC) curve analyses. Area under curve (AUC) of both models was compared using DeLong test. The dependent variable was functional independence (mRS 0–2 at day 90).
A statistically significant difference was accepted at a p value of less than 0.05. Analyses were performed using MedCalc (version 11.5.1.0; Mariakerke, Belgium) and R (R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria, 2017).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Patients
A total of 99 patients fulfilled the inclusion criteria. The median age of the patients was 76 years (IQR: 65–80). 52 (53%) patients were female and 47 (47%) were male. The median NIHSS was 16 (interquartile range (IQR): 12–19) and the initial ASPECTS was 8 (IQR: 7–9). Functional independence at day 90 (mRS 0–2) was observed in 45 patients (45%). Patient characteristics are summarized in Table 1.
All patients underwent MT with partial to complete recanalization, 12 patients with partial (mTICI 2a) (12%), 35 patients with successful but incomplete (mTICI 2b) (35%), and 52 patients (52%) with complete recanalization (16 patients with mTICI 2c, and 36 patients with mTICI3). The median time from onset to recanalization was 291 min (IQR: 233–395 min). At baseline, the median ischemic core volume was 15.6 mL (IQR: 1.2–47.5 mL), and the median penumbra volume was 213.7 mL (IQR: 175.5–265.0 mL).
Comparing patients with functional independence at day 90 (mRS 0–2) to patients with an mRS 3–6, we observed that patients with functional independence were younger (68 versus 77 years) (p < 0.01), and showed a lower NIHSS on admission (13–17) (p < 0.001). On baseline imaging, ischemic core volume was by trend lower (9–19 mL) (p = 0.71) in the patients with functional independence at day 90. Furthermore, patients with functional independence received intravenous alteplase more often (78 versus 55%) (p = 0.02), and the degree of recanalization after MT was higher (complete recanalization 71 versus 52%) (p = 0.04). Total infarct volume in FCT was lower in patients with functional independence (12–49 mL) (p < 0.01) (Table 1).
In patients with complete recanalization, the median PSV was significantly higher than the median PSV in patients with partial or incomplete recanalization (median 224 mL, IQR: 168–303 versus 158 mL, IQR: 129–225; p < 0.01). Correspondingly, the median relative penumbra salvage (proportion of rescued penumbra/total penumbral volume at baseline) was 95% (IQR: 77–99%) for patients with complete recanalization, which was significantly higher than the median relative penumbra salvage in patients with partial or incomplete recanalization (82%, IQR: 56–96%; p = 0.04).
Penumbra salvage volume—linear regression analyses
In univariable linear regression analysis, degree of recanalization, hypoperfusion volume, and penumbra volume were significantly associated with PSV as dependent parameter (Table 2). In multivariable linear regression analysis, degree of recanalization (ß = 32.1; p = 0.009), penumbra volume (per 1 mL) (ß = 0.83; p < 0.001), and time from symptom onset to recanalization (ß = − 0.11, p = 0.03), were significantly and independently associated with PSV (Table 2). There was no association between age and PSV, and no association between baseline core lesion volume and PSV.
Functional outcome—ordinal regression analyses
In univariable ordinal regression analysis, age, ASPECTS, baseline NIHSS, ischemic core volume, PSV, and degree of recanalization were significantly associated with mRS shift at day 90 as dependent parameter (Table 2, lower part). In multivariable ordinal regression analysis, PSV (odds ratio: 0.89; p < 0.0001), penumbra volume (odds ratio: 1.08; p = 0.011), ischemic core lesion volume (per mL) (odds ratio: 1.18; p < 0.001), age (odds ratio: 1.07; p < 0.001), and degree of recanalization (odds ratio: 0.41; p = 0.039) were significantly and independently associated with mRS shift at day 90 (Table 2, lower part). Time from symptom onset to recanalization, and baseline NIHSS were not significantly associated with outcome.
Multivariable prediction model
The AUC for model 1 (− PSV) to classify functional independence (mRS 0–2 at day 90) was 0.71 (95% CI: 0.60–0.80; p < 0.001). The AUC for model 2 (+ PSV) to classify functional independence was 0.80 (95% CI: 0.70–0.88; p < 0.0001). In pairwise comparison using DeLong tests, we observed a significant difference between both models (difference 0.09, 95% CI: 0.02–0.16; p = 0.015).
Discussion
Our study on the relationship of recanalization degree and PSV, and its impact on functional outcome revealed several findings: (1) higher degree of recanalization was directly associated with increased PSV: every increase in reperfusion (partial (mTICI 2a), incomplete (mTICI 2b), and complete (mTICI ≥ 2c) recanalization), was associated with a 63 mL increased PSV; (2) that was associated with improved functional outcome at 90-days follow-up; (3) this effect was shown even when comparing patients with mTICI 2b to patients with mTICI ≥ 2c.
In detail, we observed that penumbra salvage depends on three parameters: penumbra volume at baseline, degree of recanalization, and time from onset to reperfusion. However, penumbra salvage was independent from baseline ischemic core volume and ASPECTS, highlighting that penumbra may be rescued even in patients presenting with extensive stroke at admission. Furthermore, we observed that higher PSV and complete reperfusion were significantly and independently associated with improved functional outcome at 90-days. Corroborating previous studies, baseline ischemic core volume, and age had a significant impact on functional outcome [13]. Nevertheless, a higher degree of penumbra salvage might lead to a better clinical outcome even in older patients, or patients with large baseline ischemic core, as exemplified in Fig. 4 (see also supplemental figures for age effect plot) [11, 23, 24]. Interestingly, time from onset to reperfusion had no significant impact on functional outcome, illustrating that patients in the extended time window may benefit from endovascular treatment [1]. Although the impact of higher recanalization degree on functional outcome is well-established, penumbra salvage may improve outcome prediction, as exemplified by comparing two multivariable predictive models. A model that included PSV exhibited a significantly better diagnostic ability to classify functional outcome (AUC: 0.71 versus 0.80).
Penumbra salvage may be considered as a measure of success of MT, hence associated with functional outcome. Yet, the effect of endovascular treatment on clinical outcome is not completely understood. Contributing factors beyond reperfusion, including the underlying pathophysiology such as magnitude of immanent tissue injury, collateral circulation, clinical variables, or subsequent developments like recurring stroke or secondary hemorrhage, reasonably influence the clinical outcomes [11, 31]. Additionally, the effect of MT on outcome may not only be attributed to penumbral salvage, but also on reducing secondary injury volumes, in particular ischemic brain edema [8,9,10, 19, 32]. To illustrate this, we observed that complete reperfusion was associated with a penumbra salvage of 74 mL. Estimating the effect of PSV on mRS at day 90 in linear regression, a PSV of 74 mL would equal a decrease in mRS of 0.22. Complete reperfusion, however, was associated with a lower mRS of 0.95. Therefore, the effect of successful reperfusion on clinical outcomes is not comprehensively explained by penumbral salvage and may be multifactorial. Lately, it has been observed that successful MT was associated with a reduced ischemic formation of 6.3%, and improved mRS at day 90 of − 1.1 [8]. Thus, edema reduction may be an explanation of the discrepancy between outcome improvement and penumbra salvage following MT [8, 9, 26].
So far, it is well known that increasing reperfusion is directly associated with improved functional outcome [20, 21]. A recent analysis of the HERMES data observed the increasing rate of favorable outcome with increasing degree of recanalization [21]. A further recent study observed that even in the subgroup of patients with “successful” MT (i.e. mTICI ≥ 2b) the highest possible reperfusion grade should be pursued [20]. However, both studies did not discuss any pathophysiological reasons regarding the relationship of functional outcome and reperfusion degree.
To our knowledge, this is the first study that directly quantified the volume of penumbra salvage and investigated its relationship to the degree of recanalization and functional outcome. This study might help to better understand how endovascular treatment effects outcome, and how to further improve functional outcome in patients. Additionally, PSV could be tested as an imaging biomarker to compare treatment effects in ischemic stroke, as measuring infarct volume in follow-up imaging may not be an optimal parameter for this concern [5]. A previous study observed, that reduced infarct volume in follow-up imaging after MT only explained 12% of the treatment benefit [5]. However, this study did not describe baseline ischemic core volume, or penumbral volume, which might represent a major limitation of that study.
Future studies may investigate whether penumbra salvage is a better mediator of the relationship of endovascular treatment and functional outcome. Furthermore, it is important to realize that in the referred study, infarct volume was derived in follow-up imaging that has been acquired between 12 h to 2 weeks after admission. This directly impairs the interindividual comparability of lesion volumes due to the significantly ranging proportion of ischemic edema. At 24 h after onset, it has been observed that edema contributes to approximately 30% of the total lesion, while after 12 h, the mean edema proportion is around 20% [7, 17, 33]. This proportion, however, significantly varies depending on time, reperfusion treatment, and individual progression [7, 25, 27]. Consequently, future research is needed to investigate how edema-corrected lesion volumes perform as a mediator between outcome and EVT, and how these volumes may improve the comparability of treatment effects.
Limitations
Limitations of this study include the relatively small number of patients, due to rigorous inclusion criteria. The intention was to obtain a homogenous patient cohort. Patients with parenchymal hemorrhage type 2 were excluded. Future studies could investigate the relationship of PSV and secondary hemorrhage. Furthermore, there is no coherent definition of the true ischemic core and penumbra, and this concept has its natural limitations [6]. Alternative approaches could use relative cerebral blood flow to define ischemic core, but this may indicate a higher occurrence of core volume overestimation [3, 4].
Conclusion
Penumbra salvage volumes increased with higher degrees of recanalization and were significantly associated with improved functional outcome at day 90. These results further emphasize the importance of complete reperfusion as a result of EVT. Penumbra salvage was independent from baseline ischemic core volume, highlighting that penumbra may be rescued even in patients presenting with extensive ischemic core at admission.
References
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG, DEFUSE 3 Investigators (2018) Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 378:708–718
Albers GW, Marks MP, Lansberg MG (2018) Thrombectomy for stroke with selection by perfusion imaging. N Engl J Med 378:1849–1850
Bivard A, Kleinig T, Miteff F, Butcher K, Lin L, Levi C, Parsons M (2017) Ischemic core thresholds change with time to reperfusion: a case control study. Ann Neurol 82:995–1003
Bivard A, McElduff P, Spratt N, Levi C, Parsons M (2011) Defining the extent of irreversible brain ischemia using perfusion computed tomography. Cerebrovasc Dis 31:238–245
Boers AMM, Jansen IGH, Brown S, Lingsma HF, Beenen LFM, Devlin TG, Roman LS, Heo JH, Ribo M, Almekhlafi MA, Liebeskind DS, Teitelbaum J, Cuadras P, du Mesnil de Rochemont R, Beaumont M, Brown MM, Yoo AJ, Donnan GA, Mas JL, Oppenheim C, Dowling RJ, Moulin T, Agrinier N, Lopes DK, Aja Rodriguez L, Compagne KCJ, Al-Ajlan FS, Madigan J, Albers GW, Soize S, Blasco J, Davis SM, Nogueira RG, Davalos A, Menon BK, van der Lugt A, Muir KW, Roos Y, White P, Mitchell PJ, Demchuk AM, van Zwam WH, Jovin TG, van Oostenbrugge RJ, Dippel DWJ, Campbell BCV, Guillemin F, Bracard S, Hill MD, Goyal M, Marquering HA, Majoie C (2019) Mediation of the relationship between endovascular therapy and functional outcome by follow-up infarct volume in patients with acute ischemic stroke. JAMA Neurol 76:194–202
Boned S, Padroni M, Rubiera M, Tomasello A, Coscojuela P, Romero N, Muchada M, Rodriguez-Luna D, Flores A, Rodriguez N, Juega J, Pagola J, Alvarez-Sabin J, Molina CA, Ribo M (2017) Admission CT perfusion may overestimate initial infarct core: the ghost infarct core concept. J Neurointerv Surg 9:66–69
Broocks G, Faizy TD, Flottmann F, Schon G, Langner S, Fiehler J, Kemmling A, Gellissen S (2018) Subacute infarct volume with edema correction in computed tomography is equivalent to final infarct volume after ischemic stroke: improving the comparability of infarct imaging endpoints in clinical trials. Invest Radiol 53:472–476
Broocks G, Flottmann F, Hanning U, Schon G, Sporns P, Minnerup J, Fiehler J, Kemmling A (2020) Impact of endovascular recanalization on quantitative lesion water uptake in ischemic anterior circulation strokes. J Cereb Blood Flow Metab 40:437–445
Broocks G, Hanning U, Flottmann F, Schonfeld M, Faizy TD, Sporns P, Baumgart M, Leischner H, Schon G, Minnerup J, Thomalla G, Fiehler J, Kemmling A (2019) Clinical benefit of thrombectomy in stroke patients with low ASPECTS is mediated by oedema reduction. Brain 142:1399–1407
Broocks G, Leischner H, Hanning U, Flottmann F, Faizy TD, Schon G, Sporns P, Thomalla G, Kamalian S, Lev MH, Fiehler J, Kemmling A (2020) Lesion age imaging in acute stroke: water uptake in CT versus DWI-FLAIR mismatch. Ann Neurol 88:1144–1152
Campbell BCV, Majoie C, Albers GW, Menon BK, Yassi N, Sharma G, van Zwam WH, van Oostenbrugge RJ, Demchuk AM, Guillemin F, White P, Davalos A, van der Lugt A, Butcher KS, Cherifi A, Marquering HA, Cloud G, Macho Fernandez JM, Madigan J, Oppenheim C, Donnan GA, Roos Y, Shankar J, Lingsma H, Bonafe A, Raoult H, Hernandez-Perez M, Bharatha A, Jahan R, Jansen O, Richard S, Levy EI, Berkhemer OA, Soudant M, Aja L, Davis SM, Krings T, Tisserand M, San Roman L, Tomasello A, Beumer D, Brown S, Liebeskind DS, Bracard S, Muir KW, Dippel DWJ, Goyal M, Saver JL, Jovin TG, Hill MD, Mitchell PJ, HERMES Collaborators (2019) Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol 18:46–55
Dargazanli C, Fahed R, Blanc R, Gory B, Labreuche J, Duhamel A, Marnat G, Saleme S, Costalat V, Bracard S, Desal H, Mazighi M, Consoli A, Piotin M, Lapergue B, AT Investigators (2018) Modified thrombolysis in cerebral infarction 2C/thrombolysis in cerebral infarction 3 reperfusion should be the aim of mechanical thrombectomy: insights from the ASTER Trial (contact aspiration versus stent retriever for successful revascularization). Stroke 49:1189–1196
Demeestere J, Scheldeman L, Cornelissen SA, Heye S, Wouters A, Dupont P, Christensen S, Mlynash M, Albers GW, Lansberg M, Lemmens R (2018) Alberta stroke program early CT score versus computed tomographic perfusion to predict functional outcome after successful reperfusion in acute ischemic stroke. Stroke 49:2361–2367
Fiorelli M, Bastianello S, von Kummer R, del Zoppo GJ, Larrue V, Lesaffre E, Ringleb AP, Lorenzano S, Manelfe C, Bozzao L (1999) Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke 30:2280–2284
Flottmann F, Broocks G, Faizy TD, McDonough R, Watermann L, Deb-Chatterji M, Thomalla G, Herzberg M, Nolte CH, Fiehler J, Leischner H, Brekenfeld C, GSR Investigators (2020) Factors associated with failure of reperfusion in endovascular therapy for acute ischemic stroke: a multicenter analysis. Clin Neuroradiol. https://doi.org/10.1007/s00062-020-00880-8
Ganesh A, Luengo-Fernandez R, Wharton RM, Rothwell PM, Oxford Vascular Study (2018) Ordinal vs dichotomous analyses of modified Rankin Scale, 5-year outcome, and cost of stroke. Neurology 91:e1951–e1960
Gerriets T, Stolz E, Walberer M, Muller C, Kluge A, Bachmann A, Fisher M, Kaps M, Bachmann G (2004) Noninvasive quantification of brain edema and the space-occupying effect in rat stroke models using magnetic resonance imaging. Stroke 35:566–571
Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Davalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millan M, Davis SM, Roy D, Thornton J, Roman LS, Ribo M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG, HERMES Collaborators (2016) Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387:1723–1731
Harston GWJ, Carone D, Sheerin F, Jenkinson M, Kennedy J (2018) Quantifying infarct growth and secondary injury volumes: comparing multimodal image registration measures. Stroke 49:1647–1655
LeCouffe NE, Kappelhof M, Treurniet KM, Lingsma HF, Zhang G, van den Wijngaard IR, van Es A, Emmer BJ, Majoie C, Roos Y, Coutinho JM, MR CLEAN Registry Investigators (2020) 2B, 2C, or 3: what should be the angiographic target for endovascular treatment in ischemic stroke? Stroke 51:1790–1796
Liebeskind DS, Bracard S, Guillemin F, Jahan R, Jovin TG, Majoie CB, Mitchell PJ, van der Lugt A, Menon BK, San Roman L, Campbell BC, Muir KW, Hill MD, Dippel DW, Saver JL, Demchuk AM, Davalos A, White P, Brown S, Goyal M, HERMES Collaborators (2019) eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg 11:433–438
Ma H, Campbell BCV, Parsons MW, Churilov L, Levi CR, Hsu C, Kleinig TJ, Wijeratne T, Curtze S, Dewey HM, Miteff F, Tsai CH, Lee JT, Phan TG, Mahant N, Sun MC, Krause M, Sturm J, Grimley R, Chen CH, Hu CJ, Wong AA, Field D, Sun Y, Barber PA, Sabet A, Jannes J, Jeng JS, Clissold B, Markus R, Lin CH, Lien LM, Bladin CF, Christensen S, Yassi N, Sharma G, Bivard A, Desmond PM, Yan B, Mitchell PJ, Thijs V, Carey L, Meretoja A, Davis SM, Donnan GA, EXTEND Investigators (2019) Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med 380:1795–1803
McDonough R, Elsayed S, Faizy TD, Austein F, Sporns PB, Meyer L, Bechstein M, van Horn N, Nawka MT, Schon G, Kniep H, Hanning U, Fiehler J, Heit JJ, Broocks G (2020) Computed tomography-based triage of extensive baseline infarction: ASPECTS and collaterals versus perfusion imaging for outcome prediction. J Neurointerv Surg. https://doi.org/10.1136/neurintsurg-2020-016848
Meyer L, Schonfeld M, Bechstein M, Hanning U, Cheng B, Thomalla G, Schon G, Kemmling A, Fiehler J, Broocks G (2020) Ischemic lesion water homeostasis after thrombectomy for large vessel occlusion stroke within the anterior circulation: the impact of age. J Cereb Blood Flow Metab. https://doi.org/10.1177/0271678X20915792
Minnerup J, Broocks G, Kalkoffen J, Langner S, Knauth M, Psychogios MN, Wersching H, Teuber A, Heindel W, Eckert B, Wiendl H, Schramm P, Fiehler J, Kemmling A (2016) Computed tomography-based quantification of lesion water uptake identifies patients within 4.5 hours of stroke onset: a multicenter observational study. Ann Neurol 80:924–934
Nawabi J, Flottmann F, Hanning U, Bechstein M, Schoen G, Kemmling A, Fiehler J, Broocks G (2018) Futile recanalization with poor clinical outcome is associated with increased edema volume after ischemic stroke. Invest Radiol 54:282–287
Nawabi J, Flottmann F, Kemmling A, Kniep H, Leischner H, Sporns P, Schon G, Hanning U, Thomalla G, Fiehler J, Broocks G (2019) Elevated early lesion water uptake in acute stroke predicts poor outcome despite successful recanalization—when “tissue clock” and “time clock” are desynchronized. Int J Stroke. https://doi.org/10.1177/1747493019884522
Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG, DT Investigators (2018) Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 378:11–21
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL (2019) Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50:e344–e418
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL, American Heart Association Stroke Council (2018) 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49:e46–e110
Seker F, Pereira-Zimmermann B, Pfaff J, Purrucker J, Gumbinger C, Schonenberger S, Bendszus M, Mohlenbruch MA (2019) Collateral scores in acute ischemic stroke: a retrospective study assessing the suitability of collateral scores as standalone predictors of clinical outcome. Clin Neuroradiol. https://doi.org/10.1007/s00062-019-00858-1
Sporns PB, Minnerup J, Warneke N, Dziewas R, Hanning U, Berkemeyer S, Zoubi T, Heindel W, Schwindt W, Niederstadt T (2017) Impact of the implementation of thrombectomy with stent retrievers on the frequency of hemicraniectomy in patients with acute ischemic stroke. Clin Neuroradiol 27:193–197
Tipirneni-Sajja A, Christensen S, Straka M, Inoue M, Lansberg MG, Mlynash M, Bammer R, Parsons MW, Donnan GA, Davis SM, Albers GW (2017) Prediction of final infarct volume on subacute MRI by quantifying cerebral edema in ischemic stroke. J Cereb Blood Flow Metab 37:3077–3084
Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, Schellinger PD, Toni D, de Vries J, White P, Fiehler J (2019) European Stroke Organisation (ESO)—European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. https://doi.org/10.1136/neurintsurg-2018-014568
Wannamaker R, Guinand T, Menon BK, Demchuk A, Goyal M, Frei D, Bharatha A, Jovin TG, Shankar J, Krings T, Baxter B, Holmstedt C, Swartz R, Dowlatshahi D, Chan R, Tampieri D, Choe H, Burns P, Gentile N, Rempel J, Shuaib A, Buck B, Bivard A, Hill M, Butcher K (2018) Computed tomographic perfusion predicts poor outcomes in a randomized trial of endovascular therapy. Stroke 49:1426–1433
Wintermark M, Flanders AE, Velthuis B, Meuli R, van Leeuwen M, Goldsher D, Pineda C, Serena J, van der Schaaf I, Waaijer A, Anderson J, Nesbit G, Gabriely I, Medina V, Quiles A, Pohlman S, Quist M, Schnyder P, Bogousslavsky J, Dillon WP, Pedraza S (2006) Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 37:979–985
Funding
Open Access funding enabled and organized by Projekt DEAL.. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JF: Research support: German Ministry of Science and Education (BMBF), German Ministry of Economy and Innovation (BMWi), German Research Foundation (DFG), European Union (EU), Hamburgische Investitions- und Förderbank (IFB), Medtronic, Microvention, Route92, Stryker. Consultant for: Acandis, Bayer, Boehringer Ingelheim, Cerenovus, Evasc Neurovascular, MD Clinicals, Medtronic, Microvention, Penumbra, Phenox, Stryker, Transverse Medical. Stock holder: Tegus Medical. All other authors declare that they have no conflict of interest.
Ethical approval
Anonymized data was recorded in accordance with ethical review board approval and institutional review board waived informed consent. The data that support the findings of this study are available from the corresponding author upon reasonable request. The study protocol was in accordance with the declaration of Helsinki.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Broocks, G., Jafarov, H., McDonough, R. et al. Relationship between the degree of recanalization and functional outcome in acute ischemic stroke is mediated by penumbra salvage volume. J Neurol 268, 2213–2222 (2021). https://doi.org/10.1007/s00415-021-10410-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10410-2