Abstract
Objective
To perform an updated review of the literature on the neurological manifestations of COVID-19-infected patients
Methods
A PRISMA-guideline-based systematic review was conducted on PubMed, EMBASE, and SCOPUS. Series reporting neurological manifestations of COVID-19 patients were studied.
Results
39 studies and 68,361 laboratory-confirmed COVID-19 patients were included. Up to 21.3% of COVID-19 patients presented neurological symptoms. Headache (5.4%), skeletal muscle injury (5.1%), psychiatric disorders (4.6%), impaired consciousness (2.8%), gustatory/olfactory dysfunction (2.3%), acute cerebrovascular events (1.4%), and dizziness (1.3%), were the most frequently reported neurological manifestations. Ischemic stroke occurred among 1.3% of COVID-19 patients. Other less common neurological manifestations were cranial nerve impairment (0.6%), nerve root and plexus disorders (0.4%), epilepsy (0.7%), and hemorrhagic stroke (0.15%). Impaired consciousness and acute cerebrovascular events were reported in 14% and 4% of patients with a severe disease, respectively, and they were significantly higher compared to non-severe patients (p < 0.05). Individual patient data from 129 COVID-19 patients with acute ischemic stroke (AIS) were extracted: mean age was 64.4 (SD ± 6.2), 78.5% had anterior circulation occlusions, the mean NIHSS was 15 (SD ± 7), and the intra-hospital mortality rate was 22.8%. Admission to the intensive care unit (ICU) was required among 63% of patients.
Conclusion
This updated review of literature, shows that headache, skeletal muscle injury, psychiatric disorders, impaired consciousness, and gustatory/olfactory dysfunction were the most common neurological symptoms of COVID-19 patients. Impaired consciousness and acute cerebrovascular events were significantly higher among patients with a severe infection. AIS patients required ICU admission in 63% of cases, while intra-hospital mortality rate was close to 23%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the identification of the first case of severe acute respiratory syndrome (SARS) CoV-2 infection in December 2019, in Wuhan, China, the global number of confirmed COVID-19 cases is roughly 36,000,000, with about one million deaths [1]. The most characteristic symptoms of patients infected with the SARS-CoV-2 are fever, cough, shortness of breath, and acute respiratory distress syndrome. However, the spectrum of COVID-19 patients varies considerably from asymptomatic or mild-symptomatic respiratory illness, to severe pneumonia and respiratory distress that may lead to admission to intensive care units (ICU), with a risk of respiratory failure and death [2, 3]. Additionally, some patients with COVID‐19 also showed neurologic symptoms that may precede or occur in the course of the infection, as headache, anosmia, cerebrovascular disease, and impaired consciousness. Knowledge of neurovirulence of SARS-CoV-2 is progressively emerging due to the increasing evidence of neurological manifestations among these patients. Indeed, it has been demonstrated that SARS-CoV [4, 5] and MERS-CoV [6] can enter into the brain through the olfactory nerves or through hematogenous and lymphatic routes, inducing neurological diseases. Nevertheless, neurological signs and symptoms of the emerging SARS-CoV-2 remain to be elucidated.
In this updated systematic review of the literature, we investigated the frequency and type of neurological symptoms associated with SARS-CoV-2, with the aim of providing a further understanding of the potential neurological manifestations of COVID-19.
Materials and methods
Literature search
The Institutional Review Board (IRB) approval was not required because this analysis was conducted on anonymized published data from the literature. A comprehensive literature search of PubMed, Ovid EMBASE, and SCOPUS was conducted for studies published from January 2019 to October 2020. This systematic review was conducted following the PRISMA guidelines [7]. The following search terms were used in both “AND” and “OR” combinations: “COVID-19”, “SARS-CoV-2”, “coronavirus”, “neurological symptoms”, “neurological manifestations”, “nervous system”. The full search strategy is reported in Table 1. The included studies are described in Table 2. The inclusion criteria were the following: series reporting COVID-19-infected patients presenting neurological manifestations of the SARSCoV-2 infection. Exclusion criteria were the following: (1) case reports; (2) review articles; (3) studies published in languages other than English with no available English translation; and (4) in vitro/animal studies. In cases of overlapped series, only the studies with the largest number of subjects or most detailed data were included. The analysis was performed by two independent reviewers, while a third author solved discrepancies.
Outcomes
The primary objective of this study was to define the neurological manifestations of COVID-19-infected patients. The secondary objectives were: (1) to describe the frequency of associated non-neurological signs and symptoms; and (2) to define the frequency of the neurological and non-neurological manifestations among severe- and non-severe-infected patients.
Data collection
The following data were extracted: (1) neurological and non-neurological manifestations of hospitalized COVID-19 patients; (2) number of patients presenting with severe vs non-severe conditions; and (3) frequency of both neurological and non-neurological manifestations among severe and non-severe patients.
Diagnosis of infection by the SARS-Cov-2 was made based on the following: real-time reverse transcription polymerase chain reaction (RT-PCR) assay for SARS-Cov-2 in symptomatic patients; and SARS-CoV-2 RT-PCR-positive associated with viral-like pneumonia in chest CT.
The severity of COVID-19 was judged based on the following criteria: (1) three studies [8,9,10] used the Fifth Revised Trial Version of the Novel Coronavirus Pneumonia Diagnosis and Treatment Guidance [11]; (2) one study defined severe patients by those who required access to the ICU [12]; and (3) the other studies [2, 8, 13,14,15] used the following parameters: (a) respiratory distress with the respiratory rate over 30 per min; (b) oxygen saturation ≤ 93% in the resting state; and (c) arterial blood oxygen partial pressure (PaO2)/oxygen concentration (FiO2) ≤ 300 mmHg.
Quality scoring
A modified version of the Newcastle–Ottawa Scale (NOS) was used for the quality assessment of the included studies [16]. This was done by assessing the patient selection criteria, comparability of the study groups, as well as the outcome and exposure assessment based on an adapted version of the NOS (details in the Supplemental Table 1). The comparability between cases and controls was not tested, as well as the non-response rate. These were not done because of the design of the included studies that were mostly retrospective cohort studies without a control group. A star rating of 0–7 was allocated to each study based on the NOS parameters. The quality assessment was performed by two authors independently. When discrepancies arose, papers were re-examined by a third author. Studies receiving 5 or more stars are considered “high-quality”. In general, “high-quality” studies were defined based on the following criteria: (1) appropriate assessment of the investigated disease; (2) appropriate assessment of the neurological manifestations; (3) definition of severe and non-severe patients; and (4) evaluation of neurological condition in severe vs non-severe patients.
Statistical analysis
We estimated, from each cohort, the cumulative frequency and 95% confidence interval for proportion (Wilson/Brown method) for each outcome. Categorical data were described by frequency. The normality of the distribution of the qualitative variables was assessed using Shapiro–Wilk test. A variable was Gaussian distributed if p was > 0.05 at the Shapiro–Wilk test. For normal variables, mean and standard deviation (SD) were reported; for variable without a normal distribution, median and interquartile range (IQR) were used. The frequency of neurological and non-neurological symptoms was compared using the chi-square test. Differences between sub-groups of analysis were considered significant at p < 0.05. All statistical analyses, descriptive and inferential, were performed with SPSS, Version 24 (IBM, Armonk, New York).
Results
Literature review
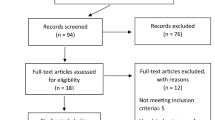
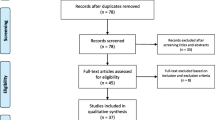
Supplemental Table 1 summarizes the included studies. The PRISMA search flow diagram is shown in Fig. 1. A total of 39 studies reporting neurological manifestations of COVID-19 patients were selected. Overall, 68,631 subjects were included.
Quality of studies
Overall, all of the included studies were retrospective case series on COVID-19-infected patients. Overall, 25 studies were designed to investigate the neurological presentation of hospitalized COVID-19 patients and were rated as “high-quality”. The other studies were designed to investigate the overall clinical characteristics of COVID-19 patients and were rated based on the availability of data on neurological manifestations (details in Supplemental Table 2).
Patient Population
Overall, 68,361 patients were included. The mean and median age of patients were 54 (SD ± 4.5) and 55 (IQR 47–58), respectively, and the proportion of male was 48% (22,322/46,460, 95% CI 47.5–48.5%).
Overall, 36.3% of patients had comorbidities (5625/15,490, 95% CI 35.5–37%). Hypertension was the most common vascular risk factor (3355/14,460 = 23.2%, 95% CI 22.5–23.9%), followed by diabetes (2055/18,120 = 11.3%, 95% CI 10.8–11.8%), history of cardiovascular disease (1205/11,585 = 10.4%, 95% CI 9.8–10.9%), and currently smoking (745/12,492 = 5.9%, 95% CI 5.5–6.4%) (details in Supplemental Table 3).
Seventy-nine percent of the COVID-19 patients presented fever (15,250/19,295, 95% CI 78.4–79.6%), 60.1% (15,470/25,100, 95% CI 60–61%) had dry cough, 31.7% fatigue (6695/21,125, 95% CI 31–32.3%), and 19.6% dyspnea (5460/27,860, 95% CI 19.1–20%). Other symptoms were anorexia, abdominal pain/nausea-vomiting, and diarrhea.
Neurological manifestations of COVID-19-infected patients
Neurological manifestations are summarized in Table 2. Overall, 21.3% (9785/45,832, 95% CI 20.9–21.7%) of COVID-19 hospitalized patients had neurological symptoms. The most common reported neurological symptoms were headache (2663/49,120 = 5.4%, 95% CI 5.2–5.6%), skeletal muscle injury (defined as myalgia or elevation of the blood creatin kinase) (2477/48,426 = 5.1%, 95% CI 4.9–5.3%), and psychiatric disorders (1892/40,986 = 4.6%, 95% CI 4.4–4.8%). Impaired consciousness (1278/45,886 = 2.8%, 95% CI 2.6–2.9%), olfactory and/or gustatory dysfunctions (998/42,561 = 2.3%, 95% CI 2.2–2.5%), acute cerebrovascular events (837/57,056 = 1.5%, 95% CI 1.37–1.57%), and dizziness (562/43,309 = 1.3%, 95% CI 5.2–5.6%) were less common neurological manifestations. Nerve root and plexus disorders, cranial nerve impairment and epilepsy represented less than 1% of the neurological symptoms.
Frequency of neurological symptoms among severe and non-severe patients
Table 3 shows the dichotomization into severe and non-severe patients that was available among ten studies. The proportion of patients with impaired consciousness was significantly higher among severe patients (176/1255 = 14%, 95% CI 12–16% vs 34/1257 = 2.7%, 95% CI 1.8–3.7%) (p = 0.0001). Similarly, acute cerebrovascular events were higher in severe patients compared to non-severe ones (28/707 = 4%, 95% CI 2.6–5.6% vs 17/873 = 1.9%, 95% CI 1.1–3%) (p = 0.02) (Table 3).
Characteristics of ischemic stroke associated with COVID-19
Among patients presenting acute cerebrovascular events, 1.3% (750/57,056%, 95% CI 1.2–1.4%) (Table 4) had acute ischemic stroke (AIS). Overall, 6 studies reported individual patient data among 129 AIS patients. The mean age of COVID-19 patients with AIS was 64.6 years (SD ± 6.2) and 41% were male. Hypertension, diabetes, atrial fibrillation, and ischemic heart disease were reported among 80%, 50%, 18%, and 26% of AIS patients, respectively. The mean delay from COVID-19 symptoms onset to AIS was 14 days (SD ± 5), and the mean NHISS at admission was 15 (SD ± 7). Anterior circulation occlusion was reported in 78.5% of cases. The intra-hospital mortality rate was 22.8%, and 63% of subjects required ICU admission.
Discussion
The clinical presentation of the SARS-CoV-2 infection is mainly associated with pulmonary complications with mild symptoms as fever, cough, dyspnea, or more severe conditions as an acute respiratory distress syndrome (ARDS) [9]. It has been reported that coronaviruses are not always confined to the respiratory tract, but they may also diffuse to the central and peripheral nervous systems, potentially inducing neurological diseases and symptoms [17].
Neurotropism has been documented for other coronaviruses: SARS-CoV has been detected in the cerebrospinal fluid (CSF) and reported as the cause of encephalitis [18]; MERS-CoV can determine severe acute disseminated encephalomyelitis and vasculopathy [6]. Because the SARS-CoV-2 is a SARS-like coronavirus, the neurovirulence has been evocated also for the new SARS-CoV-2, and a growing body of evidence is confirming this hypothesis.
Pooling 39 studies with about 68,000 patients with a laboratory-confirmed diagnosis of COVID-19, our systematic review, the largest to date, shows that 21.3% of patients may present neurological symptoms associated to the SARS-CoV-2 infection.
Headache
Headache was the most common neurological manifestation involving 5.4% of COVID-19 patients. This is a frequent neurological symptom largely reported in other diseases, such as the H1N1 influenza. In a series of H1N1 infected patients, roughly 40% of subjects had neurological manifestations, with about 35% of patients having headache [19]. In a report of neurological complications of SARS-CoV-2, Nath et al. [20] raised the question if headaches could be secondary to a viral meningitis or encephalitis in some severe cases of COVID-19 patients. Headache can be a direct para- or post-infectious consequence of COVID-19 infection, despite the fact that in our review, it was equally represented in the group with severe and non-severe disease, underlining what is probably a non-specific symptom of severe CNS involvement.
Neuropsychiatric disorders
There is growing literature showing that patients with COVID-19 may have neuropsychiatric manifestations. In a recent series, anxiety, mood disorders, emotional state symptoms, sleep disorders, and suicidal ideation have been reported in about 13% of COVID-19 patients [21]. In a nationwide, cross-specialty surveillance study of acute neurological and psychiatric complications of COVID-19, 25% were identified with altered mental status, reflecting both neurological and psychiatric diagnoses, such as encephalitis and psychosis [22]. The overall proportion of patients having psychiatric disorders was close to 5% in our review. In some studies, a disproportionate number of neuropsychiatric presentations in younger patients has been observed [22]. Some of the neuropsychiatric disorders can be associated to complications derived from an extended period in the ICU, like somnolence, confusion, and delirium. In addition, patients with a vulnerable mental status may be particularly exposed to the risk of psychiatric manifestations caused by the context of the COVID-19 pandemic [23].
Neuromuscular involvement
Neuromuscular involvement, with elevated creatine kinase or lactate dehydrogenase, was reported in about 5% of patients in our review. It is unclear whether striated muscle injury is a viral myositis or just nonspecific complications of severe viral infection, as critical illness myopathy, or polyneuropathy [24]. In the previous SARS-CoV epidemic, there were some reports of axonal polyneuropathy or myopathy [25]. In recently published studies of SARS-CoV-2 infection in China, myalgia or fatigue affected up to 70% of hospitalized patients, with about 1 out of 3 patients with increased creatine kinase (CK) [12, 15]. However, no additional work-up, such as electromyography, muscle imaging, or histopathology has been reported in these series.
In a recent report, two patients infected with severe SARS-CoV-2 acutely presented Miller Fisher syndrome and isolated multiple cranial neuropathy, respectively, suggesting a para- or post-viral process, probably related to an aberrant immune response to COVID-19 [26]. Consequently, while a possible direct infection of the virus through ACE2 receptors of skeletal muscle cells and PNS has been supposed [27], the uncontrolled immune response with elevated pro-inflammatory cytokines can be also a reasonable explanation [15].
Olfactory and gustatory dysfunctions
In our review, olfactory and/or gustatory dysfunctions were found in approximately 2.3% of patients. However, it is interesting to underline that the frequency of these symptoms is in part related to the design of the study and the investigated population. A series of 417 mild-to-moderate COVID-19 European patients, designed to investigate olfactory/gustatory disorders, reported a frequency of 85% and 88% of anosmia and gustatory dysfunctions, respectively [28]. Roughly 12% of these cases were diagnosticated before the rest of symptoms, and approximately 22% of cured patients showed persistence of both olfactory and gustatory dysfunctions [28].
Conversely, another series of Chinese patients looking for the overall frequency of neurological symptoms, reported a lower rate, with about 9% of patients having ageusia and/or hyposmia [15]. A very recent study of Spanish COVID-19 patients underlined a frequency of smell and taste disorders close to 40% [29]. According to these data, the frequency of otolaryngologic dysfunction seems to be substantially higher in European COVID-19 patients, compared to the Asian ones, but this difference needs to be investigated. Indeed, some hypotheses can be raised: either in Asian studies, the otolaryngologic complaints were not fully assessed, or the Chinese patients had a different tissue sensibility to the virus. Indeed, mutations of surface proteins (responsible for virus entry into the cell) [30] and polymorphisms of the ACE2 receptors [31] (that could be specific to certain populations) may influence susceptibility to develop certain symptoms [32]. Nevertheless, the pathophysiological mechanisms leading to the olfactory and gustatory dysfunctions are still unknown. Previous studies have shown the ability of SARS-CoV to determine neuronal death in mice by invading the brain via the olfactory epithelium [33]. Accordingly, the olfactory bulb may be a pathway of the transneuronal spread of the virus. Moreover, to date, there is no evidence that patients with anosmia or gustatory disfunction are at higher risk of a neuroinvasive SARS-CoV-2 infection [17].
Cranial nerve involvement and nerve root/plexus disorders
There are several reports describing polyradiculoneuropathy mediated by the COVID-19 infection. This could be explained by the aberrant autoimmune response targeting the peripheral and cranial nerves during the infection. Our review showed up to 0.6% and 0.4% of patients reporting nerve root and plexus dysfunctions, and cranial nerve impairment, respectively. Accordingly, some cases of Guillain–Barré syndrome have been reported associated with the SARS-CoV-2 virus [34, 35]. These cases, mostly occurring after some days or weeks after the infection, may be considered a consequence of the immune response to peripheral nerve antigens, resulting in demyelination and axonal injury. Although it should be established if COVID-19 is the trigger of Guillain–Barré disease and other peripheral nerve disorders occurring during the infection. Clinicians should be aware of the possible association between the coronavirus and these manifestations [34,35,36].
Impaired consciousness
In our analysis, impaired consciousness was reported in 2.8% of COVID-19 patients. Acute disseminated encephalomyelitis has been reported in MERS-CoV [6], and recently in SARS-CoV-2 [37]. Poyiadji et al. [37] described a case of acute necrotizing encephalopathy in a COVID-19 patient admitted with fever, cough and altered mental status. Another recent case has been published by Filatov et al. [38], reporting a 74-year-old male with COVID-19 infection presenting with altered mental status and developing an acute encephalopathy. These cases may underline the importance of a comprehensive pathogen examination in case of encephalitis- or meningitis-related symptoms among COVID-19-infected patients. Most importantly, among studies comparing severe and non-severe SARS-CoV-2 patients, impaired consciousness was significantly higher in subjects with an unfavorable evolution of the disease. About 3% of patients showing a non-severe disease presented impaired consciousness, compared to 14% of those showing an aggressive disease.
As previously reported, encephalopathy or meningitis should be checked in COVID-19 patients presenting with an altered mental status, due to the neurotropism of the virus; however, it should be investigated if deterioration of the consciousness is directly related to an involvement of the CNS in all cases or related to hypoxia/ respiratory distress [39].
Acute cerebrovascular disease
In a recent series of 221 patients infected with COVID-19 hospitalized in Wuhan, Li et al. [40] reported that roughly 5% of subjects had acute ischemic stroke, at median 10 days after symptom onset, while cerebral venous sinus thrombosis and cerebral hemorrhage were less common neurological complications (1%). Investigating the literature, the overall frequency of acute ischemic stroke among COVID-19 patients was 1.3%. Most important, about 4% of patients with a severe disease presented an acute cerebrovascular illness, compared to 1.9% of those having a favorable evolution. The mortality rate in the series of Yanan et al. [40] was close to 40% among COVID-19 patients presenting acute ischemic strokes, underlining that this condition is an important negative prognostic factor.
In a retrospective series of 1916 patients, Merkler et al. underlined a higher risk of acute ischemic strokes (approximately 1.6%) among COVID-19 compared to patients with influenza [41].
Stroke mechanisms may be multiple and can include hypercoagulability from critical illness, cardioembolism from virus-related cardiac injury, and severe inflammation [42]. In fact, the dysfunction of endothelial cells induced by infection may promote an increased thrombin generation and fibrinolysis [43]; moreover, the hypoxia found in severe COVID-19 patients can be a trigger for thrombosis, increasing blood viscosity, and inducing hypoxia-inducible transcription factors [44]. Indeed, it has been reported that ICU patients with severe respiratory disease requiring extracorporeal membrane oxygenation may experience higher rate of neurological complications, with about 13% of ischemic and hemorrhagic events, likely related to the loss of cerebral autoregulation and/or vasoconstriction following arterial pressure variation during extracorporeal membrane oxygenation [45]. According to these results, a recent study of histopathological changes of brain specimens obtained from 18 patients who died 0–32 days after the onset of symptoms of COVID-19 showed only hypoxic changes and did not show encephalitis or other specific brain changes referable to the virus [46]. In our analysis, 63% of AIS patients required ICU admission, while almost 23% experienced mortality during hospitalization. Higher levels of blood C-reactive protein, and higher d-dimer concentration [40] have be reported in SARS-CoV-2 patients with acute cerebrovascular disease, underlining a hypercoagulability state: for this reason, anticoagulant therapy has been suggested for patients presenting elevated d-dimer and fibrin degradation products [47, 48], although the optimal management of COVID-19 patients with acute cerebrovascular events remain to be elucidated [13].
Limitations
There are several study-related limitations. This study was not registered in any registration platform for systematic review and meta-analysis. In addition, several data on neurological manifestations are missing in some of the included studies because they were not designed to investigate neurological symptoms. We cannot exclude a higher frequency of most benign neurological manifestations as headache or anosmia in patients with a more favorable condition and so not hospitalized.
In addition, the reported series were retrospective studies. Although the frequency of neurological conditions among severe and non-severe patients was reported, the mortality associated with the combination of neurological and infective disease was rarely described. Finally, the definition of severe and non-severe disease was heterogeneous between studies.
Conclusion
This updated review of literature shows that headache, skeletal muscle injury, psychiatric disorders, impaired consciousness, and gustatory/olfactory dysfunctions were the most common neurological symptoms of COVID-19 patients. Impaired consciousness and acute cerebrovascular events were significantly higher among patients with a severe infection. Acute ischemic stroke patients required ICU admission in 63% of cases, while intra-hospital mortality rate was close to 23%.
References
https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 1 Oct 2020
Wan S, Xiang Y, Fang W et al (2020) Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol 92:797–806
Wang Z, Yang B, Li Q, Wen L, Zhang R (2020) Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 71:769–777
Abduljalil JM, Abduljalil BM (2020) Epidemiology, genome, and clinical features of the pandemic SARS-CoV-2: a recent view. New Microbes New Infect 35:100672
Chen J, Qi T, Liu L et al (2020) Clinical progression of patients with COVID-19 in Shanghai China. J Infect 80:e1–e6
Arabi YM, Harthi A, Hussein J et al (2015) Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV). Infection 43:495–501
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Reprint–preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther 89:873–880
Zhang J, Wang X, Jia X et al (2020) Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect 26:767–772
Chen T, Wu D, Chen H et al (2020) Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 368:m1091
Qin C, Zhou L, Hu Z et al (2020) Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis 71(15):762–768. https://doi.org/10.1093/cid/ciaa248
The fifth revised trial version of the novel coronavirus pneumonia diagnosis and treatment guidance. https://www.nhc.gov.cn/yzygj/s7652m/202002/41c3142b38b84ec4a748e60773cf9d4f.shtml. Accessed 1 Oct 2020
Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506
Wang D, Hu B, Hu C et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323:1061–1069
Deng Y, Liu W, Liu K et al (2020) Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J (Engl) 133:1261–1267
Mao L, Jin H, Wang M et al (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 77:683–690
Wells GSB, O’Connell D (2011) The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonradomized studies in meta-analyses. Ottawal Hospital Research Institute, Ottawa
Baig AM, Khaleeq A, Ali U, Syeda H (2020) Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 11:995–998
Hung EC, Chim SS, Chan PK et al (2003) Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem 49:2108–2109
Asadi-Pooya AA, Yaghoubi E, Nikseresht A, Moghadami M, Honarvar B (2011) The neurological manifestations of H1N1 influenza infection; diagnostic challenges and recommendations. Iran J Med Sci 36:36–39
Nath A (2020) Neurologic complications of coronavirus infections. Neurology 94:809–810
Nalleballe K, Reddy Onteddu S, Sharma R et al (2020) Spectrum of neuropsychiatric manifestations in COVID-19. Brain Behav Immun 88:71–74
Varatharaj A, Thomas N, Ellul MA et al (2020) Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry 7:875–882
Orsini A, Corsi M, Santangelo A et al (2020) Challenges and management of neurological and psychiatric manifestations in SARS-CoV-2 (COVID-19) patients. Neurol Sci 41:2353–2366
Pleasure SJ, Green AJ, Josephson SA (2020) The spectrum of neurologic disease in the severe acute respiratory syndrome coronavirus 2 pandemic infection: neurologists move to the frontlines. JAMA Neurol 77:679–680
Tsai LK, Hsieh ST, Chao CC et al (2004) Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol 61:1669–1673
Gutierrez-Ortiz C, Mendez-Guerrero A, Rodrigo-Rey S et al (2020) Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology 95:e601–e605
Cabello-Verrugio C, Morales MG, Rivera JC, Cabrera D, Simon F (2015) Renin–angiotensin system: an old player with novel functions in skeletal muscle. Med Res Rev 35:437–463
Lechien JR, Chiesa-Estomba CM, De Siati DR et al (2020) Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 277:2251–2261
Beltran-Corbellini A, Chico-Garcia JL, Martinez-Poles J et al (2020) Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case–control study. Eur J Neurol. https://doi.org/10.1111/ene.14273
Benvenuto D, Giovanetti M, Ciccozzi A, Spoto S, Angeletti S, Ciccozzi M (2020) The 2019-new coronavirus epidemic: Evidence for virus evolution. J Med Virol 92:455–459
Cao Y, Li L, Feng Z et al (2020) Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov 6:11
Chan JF, Yuan S, Kok KH et al (2020) A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395:514–523
Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S (2008) Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 82:7264–7275
Kilinc D, van de Pasch S, Doets AY, Jacobs BC, van Vliet J, Garssen MPJ (2020) Guillain-Barre syndrome after SARS-CoV-2 infection. Eur J Neurol 27(9): 1757–1758
Paybast S, Gorji R, Mavandadi S (2020) Guillain–Barre syndrome as a neurological complication of novel COVID-19 infection: a case report and review of the literature. Neurologist 25:101–103
Tatu L, Nono S, Gracio S, Kocer S (2020) Guillain-Barre syndrome in the COVID-19 era: another occasional cluster? J Neurol. https://doi.org/10.1007/s00415-020-10005-3
Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B (2020) COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology 296:E119–E120
Filatov A, Sharma P, Hindi F, Espinosa PS (2020) Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus 12:e7352
Lu L, Xiong W, Liu D et al (2020) New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia 61:e49–e53
Li Y, Li M, Wang M et al (2020) Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol 5:279–284
Merkler AE, Parikh NS, Mir S et al (2020) Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2020.2730
Guo T, Fan Y, Chen M et al (2020) Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 5:811–818
Schmitt FCF, Manolov V, Morgenstern J et al (2019) Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann Intensive Care 9:19
Gupta N, Zhao YY, Evans CE (2019) The stimulation of thrombosis by hypoxia. Thromb Res 181:77–83
Sutter R, Tisljar K, Marsch S (2018) Acute neurologic complications during extracorporeal membrane oxygenation: a systematic review. Crit Care Med 46:1506–1513
Solomon IH, Normandin E, Bhattacharyya S et al (2020) Neuropathological features of Covid-19. N Engl J Med 383:989–992
Tang N, Bai H, Chen X, Gong J, Li D, Sun Z (2020) Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 18:1094–1099
Tang N, Li D, Wang X, Sun Z (2020) Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18:844–847
Qin C, Zhou L, Hu Z et al (2020) Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis 71:762–768
Chen N, Zhou M, Dong X et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395:507–513
Jin X, Lian JS, Hu JH et al (2020) Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 69:1002–1009
Guan WJ, Liang WH, Zhao Y et al (2020) Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 55:2000547
Lian J, Jin X, Hao S et al (2020) Analysis of epidemiological and clinical features in older patients with coronavirus disease 2019 (COVID-19) outside Wuhan. Clin Infect Dis 71:740–747
Beyrouti R, Adams ME, Benjamin L et al (2020) Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry 91:889–891
Oxley TJ, Mocco J, Majidi S et al (2020) Large-Vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med 382:e60
Karadas O, Ozturk B, Sonkaya AR (2020) A prospective clinical study of detailed neurological manifestations in patients with COVID-19. Neurol Sci 41:1991–1995
Chougar L, Shor N, Weiss N et al (2020) Retrospective observational study of brain magnetic resonance imaging findings in patients with acute SARS-CoV-2 infection and neurological manifestations. Radiology. https://doi.org/10.1148/radiol.2020202422
Kremer S, Lersy F, de Seze J et al (2020) Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology 297(2):E242–E251
Xiong W, Mu J, Guo J et al (2020) New onset neurologic events in people with COVID-19 in 3 regions in China. Neurology 95:e1479–e1487
Kremer S, Lersy F, Anheim M et al (2020) Neurologic and neuroimaging findings in patients with COVID-19: a retrospective multicenter study. Neurology 95:e1868–e1882
Romero-Sanchez CM, Diaz-Maroto I, Fernandez-Diaz E et al (2020) Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology 95:e1060–e1070
Jain R, Young M, Dogra S et al (2020) COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci 414:116923
Benussi A, Pilotto A, Premi E et al (2020) Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia, Lombardy, Italy. Neurology 95:e910–e920
Mahammedi A, Saba L, Vagal A et al (2020) Imaging in neurological disease of hospitalized COVID-19 patients: an italian multicenter retrospective observational study. Radiology 297(2):E270–E273. https://doi.org/10.1148/radiol.2020201933
Garcia-Monco JC, Cabrera-Muras A, Collia-Fernandez A et al (2020) Neurological reasons for consultation and hospitalization during the COVID-19 pandemic. Neurol Sci. https://doi.org/10.1007/s10072-020-04714-w
Jalessi M, Barati M, Rohani M et al (2020) Frequency and outcome of olfactory impairment and sinonasal involvement in hospitalized patients with COVID-19. Neurol Sci 41:2331–2338
Lechien JR, Cabaraux P, Chiesa-Estomba CM et al (2020) Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck 42:1583–1590
Martin-Sanchez FJ, Del Toro E, Cardassay E et al (2020) Clinical presentation and outcome across age categories among patients with COVID-19 admitted to a Spanish Emergency Department. Eur Geriatr Med. https://doi.org/10.1007/s41999-020-00359-2
Cummings MJ, Baldwin MR, Abrams D et al (2020) Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. medRxiv. https://doi.org/10.1101/2020.04.15.20067157
Rothstein A, Oldridge O, Schwennesen H, Do D, Cucchiara BL (2020) Acute cerebrovascular events in hospitalized COVID-19 patients. Stroke 51:e219–e222
John S, Kesav P, Mifsud VA et al (2020) Characteristics of large-vessel occlusion associated with COVID-19 and ischemic stroke. AJNR Am J Neuroradiol. https://doi.org/10.3174/ajnr.A6799
Annie F, Bates MC, Nanjundappa A, Bhatt DL, Alkhouli M (2020) Prevalence and outcomes of acute ischemic stroke among patients ≤ 50 years of age with laboratory confirmed COVID-19 infection. Am J Cardiol 130:169–170
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
FC participated in literature search, study design, data collection, data analysis, and interpretation of results and wrote the manuscript. CA participated to the study design, writing the manuscript, interpretation of the e results. IM, CD, PH, VC, CR, AB, GG, NG, IM participated in the data interpretation. FC participated in statistical methodology and data interpretation. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest.
Ethical approval
Ethical approval was not required because it is a review of the literature.
Informed consent
Written informed consent was not required because it was a review of the literature with anonymous data.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cagnazzo, F., Arquizan, C., Derraz, I. et al. Neurological manifestations of patients infected with the SARS-CoV-2: a systematic review of the literature. J Neurol 268, 2656–2665 (2021). https://doi.org/10.1007/s00415-020-10285-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-10285-9