Abstract
We performed a meta-analysis to synthesize evidence on the efficacy and safety of physical exercise as an add-on therapeutic intervention for quality of life (QoL), depressive symptoms and cognition across six chronic brain disorders: Alzheimer’s disease, Huntington’s disease, multiple sclerosis, Parkinson’s disease, schizophrenia and unipolar depression. 122 studies ( = k) (n = 7231) were included. Exercise was superior to treatment as usual in improving QoL (k = 64, n = 4334, ES = 0.40, p < 0.0001), depressive symptoms (k = 60, n = 2909, ES = 0.78, p < 0.0001), the cognitive domains attention and working memory (k = 21, n = 1313, ES = 0.24, p < 0.009), executive functioning (k = 14, n = 977, ES = 0.15, p = 0.013), memory (k = 12, n = 994, ES = 0.12, p = 0.038) and psychomotor speed (k = 16, n = 896, ES = 0.23, p = 0.003). Meta-regression showed a dose–response effect for exercise time (min/week) on depressive symptoms (β = 0.007, p = 0.012). 69% of the studies that reported on safety, found no complications. Exercise is an efficacious and safe add-on therapeutic intervention showing a medium-sized effect on QoL and a large effect on mood in patients with chronic brain disorders, with a positive dose–response correlation. Exercise also improved several cognitive domains with small but significant effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic brain disorders are associated with reduced quality of life (QoL) [1,2,3,4], high prevalence of low mood and depression, stress sensitivity and cognitive dysfunction [5, 6]. These sequelae are interdependent, as depressive mood and cognitive impairment are two main factors influencing QoL [1, 2, 4,5,6,7,8], while cognition is negatively influenced by depression [9]. Moreover, these general sequelae are associated with various adverse consequences such as poor treatment compliance, loss of independence and even mortality [10]. In treatment of brain disorders, current clinical practice tends to focus on improving disease-specific symptoms (e.g., tremor and rigidity in Parkinson’s disease, psychosis in schizophrenia). Notably, however, patients with brain disorders regard QoL and depressive mood as more important for their health status than disease-specific physical and mental symptoms [11]. Therefore, improvement of these common features should become an important target in treatment of chronic brain disorders.
Exercise therapy may positively affect QoL, depression and cognition across disorders. A leading example is stroke, in which physical exercise has shown favorable effects in improving a wide range of symptoms, such that it has now been incorporated and recommended in guidelines as part of the standard treatment [12,13,14,15,16]. In contrast, research on the efficacy of physical exercise in treatment of other brain disorders is still in its infancy and therefore not part of the standard care. Although several studies have investigated the effect of physical exercise in different chronic brain disorders such as Alzheimer’s disease (AD) [17, 18], multiple sclerosis (MS) [19,20,21], Parkinson’s disease (PD) [22, 23], Schizophrenia (Sz) [24, 25] and unipolar depression (UD) [26,27,28], results and mainly recommendations for clinical practice have been highly diverse [29]. As a consequence, current evidence for efficacy of exercise therapy is still disputed and exercise is not part of the regular care offer for patients with aforementioned disorders in most countries.
Of note, the above-mentioned chronic brain disorders share underlying pathophysiological mechanisms. As such, neuroinflammation [30,31,32,33], imbalance in same neurotransmitter (e.g., dopamine in Sz and PD [34, 35], serotonin in Sz and UD [36]) and growth factors (e.g., brain-derived neurotrophic factor; BDNF) [37, 38], and disturbed connectivity (e.g., in default-mode network) [39,40,41,42] have been implicated in the pathophysiology of many of these brain disorders. Furthermore, a recent genome-wide association study (GWAS) showed high degree of genetic overlap among many psychiatric disorders stating that the different psychiatric disorders do not reflect independent diseases but rather represent different overlapping phenotypes of the same clinical spectra [43].
The aforementioned shows how disease-specific research has de-emphasized and limited our understanding of substantial commonalities that exist across disorders. Considering the overlap in pathophysiology and clinical picture across chronic brain disorders, commonalities across disorders outweigh the differences indicating that transdiagnostic and disease-specific treatments might be at least equally effective. Therefore, by targeting the common functional relationships across disorders with transdiagnostic treatments, both disease-specific and common shared factors can be targeted during treatment. Physical exercise can be such a transdiagnostic treatment for chronic brain disorders.
The objective of this study is to quantitatively review the effect of additional physical exercise on QoL, depressive symptoms and cognition across the above-mentioned disorders. In addition, we aim to estimate the safety of exercise in aforementioned groups. There are of course more chronic brain disorders in which exercise therapy may be effective, but for reasons of feasibility we restricted this review to six different brain disorders of various origins.
Method
Literature search
This meta-analysis was performed according to the Preferred Reporting for Systematic Reviews and Meta-analysis (PRISMA) Statement [44]. A systematic search was performed in Pubmed (Medline), Embase, PsychInfo and Cochrane Database of Systematic Reviews (independently by MD, MS, and EL), using combinations of the following search terms: ‘Alzheimer’, ‘AD’, ‘Huntington’, ‘HD’, ‘multiple sclerosis’, ‘MS’, ‘Parkinson’, ‘PD’, ‘PDD’, ‘schizophrenia’, ‘psychosis’, ‘psychotic’, ‘depression’, ‘depressive’, ‘mood’, ‘affective’, ‘exercise’, ‘physical’, ‘training’, ‘endurance’, ‘aerobic’, ‘anaerobic’, ‘resistance’, ‘sport’ and ‘yoga’ (Online Resource 1), with no year or language limits. Additionally, the Web of Sciences databases and review articles were examined for cross-references. The search cutoff date was 15th of September 2018. When necessary, corresponding authors were contacted to provide full text details of the study outcome measures.
Inclusion criteria
By consensus (between MD, MS, EL, and IS), the following studies were included:
-
1.
Randomized controlled trials (RCTs) investigating the effect of any type of physical exercise as an add-on intervention on QoL, depressive symptoms and/or cognition
-
2.
Studies investigating whole-body, or upper- or lower-body exercise (i.e., organ-specific exercise such as respiration muscle or pelvic muscle training were excluded)
-
3.
Studies including patients with a diagnosis of AD, HD, MS (idiopathic) PD, Sz [24] and UD (according to a diagnostic interview) in both the intervention and control group (i.e., mixed study populations were excluded)
-
4.
RCTs with a cross-over design providing data for the first study period
-
5.
Studies investigating combined interventions when the control group received the same non-exercise component of the intervention (e.g., exercise + medication versus medication only)
-
6.
Studies investigating rehabilitation programs, provided that physical exercise constituted a main part of the program
-
7.
Studies reported sufficient information to compute common effect size (ES) statistics [i.e., mean and standard deviations (SDs), exact F, p, t, or z values] or corresponding authors could provide these data upon request
-
8.
If multiple publications were retrieved that described the same cohort, only the sample with largest overall sample size and/or original data was included
Exclusion criteria
-
1.
Studies investigating same type of physical exercise in both the intervention and control group
-
2.
Abstracts of studies (without full-text available) with insufficient information about the physical exercise intervention and/or outcome measures to calculate ES and untraceable corresponding information of the authors
Outcome measures
The outcome measures included pre- and post-intervention assessments (i.e., measured directly after finishing the intervention and thus does not include follow-up measurements) of QoL, depressive symptom severity and/or cognition. For measurements of depressive symptoms, observer-rated scales were preferred over self-rated questionnaires because of its higher validity [45]. The scales used to measure depression comprised Hamilton Depression Rating Scale (HDRS) [46], Beck Depression Inventory (BDI) [47], Montgomery Asberg Depression Rating Scale (MADRS) [48], Geriatric Depression Scale (GDS) [49], Patient Health Questionnaire-9 (PHQ-9) [50], and Profile of Mood States (POMS) [51].
Based on the cognitive domains and/or cognitive tests investigated across studies and disorders, the following six cognitive domains were classified: attention and working memory (A&WM), executive functioning (EF), memory (M), psychomotor speed (PS), verbal fluency (VF) and global cognition (GC) (Online Resource 2). To combine studies across disorders, the most stringent control group per disorder [i.e., treatment as usual (TAU) allowing treatments such as disease-specific medication, reading newspapers, educational sessions but no active treatments such as occupational therapy] was used as a reference group.
Assessment of risk of bias
According to the Cochrane Handbook of Systematic Reviews of Interventions [52], risk of bias was assessed for all eligible studies regarding selection bias, detection bias, attrition bias and reporting bias. Attrition bias was divided into assessment of incomplete outcome data (i.e., drop-out and exclusions) and intention-to-treat (ITT) analysis as ITT is considered the least biased method to measure intervention effects in RCTs [52]. Performance bias was not assessed, as it is usually not possible to blind study participants to whether or not exercise intervention is performed.
Data analysis
All analyses were performed using Comprehensive Meta-Analysis Version 2.0. Per outcome measure, the effect of additional exercise (versus control group) was quantified for each study using Hedges’ g based on change scores (end of treatment minus baseline). When these were not reported, pre- and post-treatment mean values and SDs, or exact F, p, t, or z values were used. For studies that did not report exact SDs, these were calculated using the 95% confidence intervals (SD = sqrt(N) × [upper limit-lower limit]/[2 × 1.96]) or standard error (SE) (SD = SE × sqrt(N)).
To achieve a single pair-wise comparison between exercise and TAU, if a study investigated two or more types of exercise intervention, groups were combined for the main analysis [53] but studied separately in the moderator analysis (see further). The ES of the individual intervention groups were combined to calculate a composite ES by incorporating the ES and variance of each individual intervention while taking into account the correlation among the different interventions [54]. Likewise, when a study used more than one questionnaire to measure QoL or depressive symptoms, or multiple neuropsychological tests to measure a cognitive domain, a composite ES was calculated. As the correlation among interventions or test measures was mostly not reported, a correlation of 0.5 was taken for all the computations to avoid under- and overestimation of the overall ES [54].
Studies were combined in meta-analysis to calculate a mean weighted ES for each outcome measure (see Online Resource 3 for formulas). A random-effects model was considered appropriate given the heterogeneity across studies and diagnoses. Moreover, a random-effects model allows generalization of the results on population level [55]. ES were interpreted according to Cohen [56], with an ES of 0.2 indicating a small effect, 0.5 a medium and ≥ 0.8 a large effect. First, analyses were performed including all suitable studies per outcome measure. Subsequently, analyses were repeated by excluding outlier studies, defined as studies with standardized residual z scores of ES exceeding ± 1.96 (p < 0.05, two-tailed; shown in Figs. 2, 3, 4), studies with small total sample sizes (n < 20) because of high risk of sampling error in effect estimates [57] and studies with high risk of bias (i.e., considering the aim of the meta-analysis to study RCTs, studies classified as having high risk of bias on randomization and allocation concealment were excluded). ES with p < 0.05 were considered significant. Heterogeneity of results across studies was assessed by calculating the Q-statistic and I2-statistic. Q-Statistic tests the existence of heterogeneity and displays a Chi-square distribution with k−1 degrees of freedom (k = number of studies). Q values higher than the degrees of freedom indicate significant between-studies variability. I2 describes the percentage of total variation across studies due to heterogeneity rather than chance. I2 values of 25%, 50%, and 75% are considered as low, moderate, and high heterogeneity, respectively [58].
Potential publication bias was investigated by visual inspection of the funnel plots, with asymmetrical funnel plots indicating publication bias. When appropriate, the funnel plot asymmetry was tested with Egger’s test (p < 0.05, two-tailed) [59]. Additionally, Rosenthal’s fail-safe number (NR) was calculated for significant ES, estimating the number of unpublished studies with non-significant results needed to bring the observed result to non-significance [60].
Moderator analyses
Subgroup analyses were performed for ‘type of exercise’ classified as aerobic, resistance, or neuromotor exercise (e.g., yoga) according to the American College of Sports Medicine (ACSM) Guideline [61].
Since an insufficient number of studies examined the effect of flexibility exercise only, analysis was not feasible for this type of exercise.
Random effects meta-regression analyses were conducted to evaluate the effect of the following continuous moderator variables using the unrestricted maximum likelihood model:
-
Exercise time (min/week)
-
Total length of the intervention period (weeks)
-
Age (overall mean age across study groups per study)
If a study reported a range for any of these variables, the mean value of the variable was calculated from the upper and lower bounds. To include each pair-wise comparison separately in these sensitivity analyses, for studies with multiple intervention groups but one shared control group, the total number of participants in the control group were evenly divided up among the comparisons [53].
Since a large number of the included studies did not provide sufficient information about the intensity and safety of the exercise intervention and most of the included studies (80%) investigated supervised exercise intervention, a sub- or meta-regression analysis was not possible to investigate the effect of these parameters. The intensity and safety of the exercise interventions were assessed qualitatively.
Results
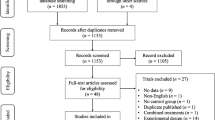
A total of 400 articles investigating the effect of any type of exercise intervention for patients with chronic brain disorders were retrieved from the literature search (AD: k = 40, HD: k = 6, MS: k = 137, PD: k = 124, Sz: k = 29, UD: k = 64), see Fig. 1.
A descriptive overview of these studies is provided in Online Resource 4. Of these, 163 studies fulfilled the inclusion criteria and were eligible for meta-analysis [62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224]. Forty-one studies provided insufficient information to compute common effect size. Therefore, a final total of 122 studies could be combined in meta-analysis. Risk of bias of all the eligible studies is shown in Online Resource 5 with a corresponding elaborative assessment of the studies included in the meta-analysis.
Quality of life
Sixty-four studies (n = 4334) examined the effect of exercise on QoL. Exercise showed a significant medium-size effect (ES = 0.40, 95% CI 0.27–0.52, p < 0.0001; Fig. 2, Table 1). Heterogeneity was high [Q(63) = 250.18, p < 0.0001; I2 = 75%], indicating that 75% of the dispersion seen in Fig. 2 reflects difference in the true effect sizes while the remaining 25% can be attributed to random sampling error. Five studies [68, 142, 186, 200, 217] were identified as outliers, six studies [68, 119, 173, 200, 208, 216] had small sample sizes (n < 20) and another four studies [135, 140, 165, 193] were classified as having high risk of bias. After exclusion, ES decreased, but remained significant (k = 51, n = 3895, ES = 0.31, 95% CI 0.19–0.43, p < 0.0001). Heterogeneity decreased, but remained moderate to high [Q(50) = 159.13, p < 0.0001; I2 = 69%]. Funnel plot and Egger’s test indicated potential publication bias before [t(62) = 5.00, p < 0.0001, NR = 1898], and after exclusion of the studies [t(49) = 3.39, p < 0.010, NR = 847] but with very high fail-safe numbers (Table 1).
Within-disorder analysis showed a positive effect of exercise on QoL in patients with MS, PD and Sz (Table 2).
Depressive symptoms
Sixty studies (n = 2909) showed a significant large-size effect of exercise on depressive symptoms (ES = 0.78, 95% CI 0.58–0.98, p < 0.0001; Fig. 3), with high heterogeneity [Q(59) = 367.90, p < 0.0001; I2 = 84%; Table 1]. Excluding eight outliers [75, 101, 104, 108, 112, 159, 220, 221], seven small studies (n < 20) [68, 82, 87, 95, 190, 207, 225] and two studies [99, 193] with high risk of bias decreased the overall ES to a medium effect (k = 43 n = 2430, ES = 0.47, 95% CI 0.32–0.62, p < 0.0001). Heterogeneity reduced to moderate to high [Q(42) = 130.55, p < 0.0001; I2 = 68%]. Funnel plot and Egger’s test indicated potential publication bias [t(58) = 6.10, p < 0.0001, NR = 3937], which remained after exclusion of the outliers [t(41) = 3.97, p < 0.001, NR = 1088; Table 1].
Within-disorder analysis showed a positive effect of exercise on depressive symptoms in AD, MS, Sz and UD (Table 2).
Cognition
Of the 120 studies, 36 studies (AD: k = 12, HD: k = 3, MS: k = 7, PD: k = 7, Sz: k = 3, UD: k = 4), examining 2125 patients, evaluated cognitive functioning and were included.
Attention and working memory
Exercise showed a significant small effect on attention and working memory (k = 21, n = 1313, ES = 0.24, 95% CI 0.06–0.41, p = 0.009; Fig. 4) with moderate heterogeneity [Q(20) = 40.83, p = 0.004; I2 = 51%]. Eight (40%) out of 20 studies comprised AD, HD or PD. The funnel plot and Egger’s test indicated potential publication bias [t(19) = 2.14, p = 0.046, NR = 55] (Table 1). The ES remained significant after excluding one outlier study [219], four small studies (n < 20) [163, 181, 190, 225] and one study [193] with high risk of bias (k = 14, n = 923, ES = 0.25, 95% CI 0.08–0.42, p = 0.004). Heterogeneity turned low to moderate [Q(13) = 20.83, p = 0.076; I2 = 38%]. Egger’s test was non-significant (Table 1).
Meta-analysis of the effect of physical exercise on the cognitive domains (from top to down) attention and working memory, executive functioning, memory, psychomotor speed, verbal fluency and global cognition. Effect sizes (ES) per study and the overall ES are in Hedges’ g with corresponding p values and sample size of the intervention and control group. Standardized residual z scores of ES were used to detect outlier studies
Executive functioning
Fourteen studies (n = 977) showed a significant small effect of exercise on executive functioning (ES = 0.15, 95% CI 0.03–0.27, p = 0.013; Fig. 4). Five (35.7%) out of 14 studies investigated physical exercise in AD, HD or PD. Studies were homogenous [Q(13) = 12.30, p = 0.503; I2 = 0%]. Egger’s test was non-significant (Table 1). After excluding one outlier [63] and three small studies [68, 163, 190], ES remained significant (k = 10, n = 916, ES = 0.17, 95% CI 0.04–0.29, p = 0.009). There were no studies with high risk of bias.
Memory
Twelve studies (n = 994) examined the effect of physical exercise on memory and showed a beneficial small effect of exercise (involving mainly aerobic exercise) (ES = 0.12, 95% CI 0.07–0.24, p = 0.038; Fig. 4). Four (33.3%) out of 2 studies comprised AD, HD or PD. Studies were homogenous [Q(11) = 10.74, p = 0.465; I2 = 0%]. Egger’s test was non-significant (Table 1). After excluding one outlier study [128] and one small study [225], ES was non-significant (k = 9, n = 939, ES = 0.09, 95% CI − 0.03 to 0.21, p = 0.127), while studies remained homogenous (Table 1).
Psychomotor speed
Exercise showed a significant small effect on psychomotor speed (k = 16, n = 896, ES = 0.23, 95% CI 0.08 to 0.38, p = 0.003; Fig. 4). Five (31.3%) out of 16 studies were based on AD, HD or PD. Heterogeneity among studies was low [Q(15) = 19.02, p = 0.213; I2 = 21%]. Funnel plot and Egger’s test indicated potential publication bias [t(14) = 2.36, p = 0.035, NR = 42]. After excluding one outlier [65] and four small studies [162, 163, 190, 225], ES remained significant (k = 10, n = 786, ES = 0.14, 95% CI 0.005–0.27, p = 0.042). Studies showed complete homogeneity and Egger’s test was non-significant (Table 1).
Verbal fluency
Exercise showed no significant effect on verbal fluency (k = 6, n = 540, ES = 0.24, 95% CI − 0.07 to 0.55, p = 0.134; Fig. 4) and remained non-significant after excluding one outlier study [65] (k = 5, n = 510, ES = 0.06, 95% CI − 0.15 to 0.27, p = 0.569). Four (66.7%) out of six studies comprised AD, HD or PD. Heterogeneity among studies was moderate to high [Q(5) = 14.36, p = 0.014; I2 = 65%; Table 1] but decreased after excluding the outlier (Table 1).
Global cognition
Fifteen studies (n = 725), all comprising AD, HD or PD, showed a trend of exercise in improving global cognition (ES = 0.30, 95% CI − 0.03 to 0.63, p = 0.076; Fig. 4). ES increased and showed significance (k = 10, n = 620, ES = 0.39, 95% CI 0.09–0.68, p = 0.010) after excluding two outliers [63, 74], three small studies [68, 119, 190] and one study [193] with high risk of bias. Heterogeneity was high [Q(14) = 60.79, p < 0.0001; I2 = 77%] but decreased after exclusion of the studies [Q(9) = 26.15, p = 0.002; I2 = 66%]. Egger’s test was non-significant (Table 1).
Separate analyses per disorder showed beneficial effects of exercise on A and WM in PD, PS in Sz and on GC in AD and PD (Table 2).
The study by Oertel Knöchel et al. [105] and Maci et al. [68] investigated physical exercise in combination with a cognitive intervention. Exclusion of these studies did not change results for any of the outcome measures.
Studies with ITT-analysis
Additional analyses with studies with only low or unclear risk of bias on ITT analyses showed even larger effect of exercise on both QoL (ES = 0.56) and depressive symptoms (ES = 0.90), while effect on the cognitive domain psychomotor speed remained small (ES = 0.24) but significant. Effect of physical exercise on all the other cognitive domains was no longer significant. See Online Resource 6 for a detailed overview of these results.
Moderator analysis
Subgroup analysis showed a significant medium effect of aerobic and neuromotor exercise and a medium-to-large effect of resistance exercise on QoL and depressive symptoms. Furthermore, a comprehensive program including all types of exercises according to ACSM was also effective in improving QoL. For cognition, aerobic and neuromotor exercises showed significant effects (Table 1).
Meta-regression analysis showed a small but positive dose–response effect for the amount of weekly exercise in min/week in reducing depressive symptoms (β = 0.007, 95% CI 0.002–0.013, p = 0.012; Online Resource 7–8), indicating that every 1-min increase in exercise intervention per week corresponds to an 0.007 unit increase is ES. No significant effect was found for the moderator total length of intervention (range 1.4–104 weeks). Additional meta-regression results are shown in Online Resource 7.
Intensity
With regard to intensity of the exercise intervention as possible moderator, 50 of the analyzed studies (41.0%) did not report any information. Of the remaining 59.0%, 18 studies (25.0%) investigated neuromotor exercises and therefore possibly could not report any intensity level. 36 studies (50.0%) applied low-to-moderate intensity of exercise, while 16 studies (22.2%) investigated moderate-to-high intensity exercise. Two studies (2.8%) investigated low-to-high intensity exercise (Online Resource 9).
Safety
Sixty-five studies (53.3%) reported on safety aspects of the exercise intervention (Online Resource 10). Forty-five of these studies (69.2%) found no physical injuries related to exercise. Eighteen studies (27.7%) found physical injuries that were related to the exercise intervention. These consisted mainly of muscle/joint pain (17.5%), fall incidents (11.4%, all with complete recovery) and ankle sprain (1.9%). In 83.3% of these studies (k = 15), physical injuries were short-lasting and/or had no consequences for participation in and completion of the exercise intervention.
Discussion
One hundred and twenty-two studies, including 7231 patients, showed a significant medium-size effect (ES = 0.40) of exercise as an add-on therapeutic intervention on QoL (k = 64, n = 4334), a large effect (ES = 0.78) on depressive symptoms (k = 60, n = 2909) and a small but significant effect (ES = 0.12–0.24) on improving function in several cognitive domains. The effects for QoL and depression were well powered. The included number of patients was lower for cognition (k = 36, n = 2125), which makes these results more sensitive for new findings. From the studies that reported on safety (k = 18), low incidences of complications related to the exercise interventions were found, which had no lasting consequences for participation in and completion of the exercise interventions.
Current clinical practice
In present clinical practice, the role of physical exercise as an add-on therapy in the management of QoL, depressive symptoms and cognitive impairment in chronic brain disorders remains elusive [226,227,228]. Management guidelines sometimes suggest physical exercise in treatment of, e.g., physical health, motor symptoms, falls and fatigue in chronic brain disorders but lack in clarity over the effectiveness of physical exercise on the studied symptoms [229,230,231,232,233,234,235].
Chronic brain disorders commonly affect well being and QoL. Therefore, improvement of QoL is a main care objective in these disorders. Depressed mood and cognitive inabilities are important contributors to reduce QoL. Currently, evidence for treatment designed specifically to target QoL is lacking. Most treatments for chronic brain disorders alleviate disease-specific symptoms, progression or relapse. In contrast, exercise therapy targets overall well-being, mood and cognition, independent of type of disease.
At present, physical exercise is not generally viewed as an effective intervention. For example, in a recent review, Kok et al. evaluated treatment of depression in older adults and stated that depressive symptoms can be effectively treated with antidepressants whereas physical exercise may not be a mainstream treatment modality, yet might be considered as a complementary therapy [236]. In contrast, Turner et al., showed that the efficacy of antidepressants is subject to selective publication of positive studies with a precipitous drop in ES to an overall ES of 0.32 when non-published FDA approved drug trials of antidepressants were combined with published drug trials [237].
For dementia, there are still no disease-modifying agents available and treatment is limited to amelioration of symptoms [238]. The effects for cognition found in our meta-analysis are statistically small but significant and similar or larger than effects of cognitive therapy [239,240,241,242,243,244] or drug treatment [245,246,247,248], which makes these effects relevant for cognitive outcomes.
Heterogeneity and moderators
To our knowledge, this is the first meta-analysis to assess the effect of physical exercise interventions across chronic brain disorders. Since heterogeneity between studies is a valid reason of concern in meta-analyses, our study shows that when we consider brain disorders to share underlying mechanisms, it is feasible to combine disorders and studies across disorders in a joint analysis. We found lower heterogeneities in the joint analysis compared to within-disorder analysis. High heterogeneity across studies and disorders was accounted for using the random-effects model and excluding outlier studies, small studies and studies with high risk of bias. As a consequence, for QoL and depressive symptoms, both heterogeneity and ES decreased, but exercise still showed a significant medium effect. Moderator analyses, performed to assess potential sources of heterogeneity, showed moderate variability between studies that investigated aerobic exercises whereas studies that evaluated the efficacy of resistance or neuromotor exercises on QoL and depressive symptoms showed higher ES and no heterogeneity. Largest effects were found for resistance exercise. Better performance of resistance exercise on these outcomes might be mediated by an increase in peripheral blood levels of Insulin-growth-factor-1 (IGF-1), which can cross the blood–brain barrier and has been shown to regulate the effects of exercise on depression, learning, angiogenesis and hippocampal neurogenesis [249, 250]. As one study evaluated the role of resistance exercise only on cognition, this result should be interpreted with caution. Heterogeneity across studies assessing cognition was low or completely lacking for all but two cognitive domains (i.e., attention and working memory and global cognition) that showed significant results. For cognition, neuromotor exercise resulted in higher effects than aerobic exercise. Neuromotor exercises involve multifaceted exercises that target different brain systems involved in the regulation of attention, balance, coordination, mood, motor functioning and cognition, amongst others. Hence, neuromotor exercises are suggested to improve synchronization between different brain areas, which might explain their efficacy on a wide variety of clinical symptoms [251].
We found a positive dose–response effect for the weekly time spent on exercise in min/week in reducing depressive symptoms, indicating that the more time spent on exercise per week, the larger the reduction in depressive symptoms. However, no significant dose–response effect was found for the total length of the exercise intervention (i.e., the number of weeks spend on exercise), suggesting that both short- and long-term exercise interventions might be beneficial in improving QoL, depressive symptoms, and cognition. Patient groups ranged in mean age from 15.4 to 84.0 years, but no significant effect of this moderator was found on the outcome measures indicating that the effect of exercise on the examined outcome measure is not age-dependent.
Regarding exercise intensity, most of the studies that provided information on the intensity of the studied exercise intervention, applied moderate exercise intensity. Additionally, we found that risk of possible complications due to exercise is low, which should not be considered a limiting factor for exercise intervention.
While all aforementioned moderators were expected to be an explanatory factor for the high heterogeneity in QoL, depressive symptoms and the cognitive domain global cognition, the role of exercise intensity and safety could not be assessed quantitatively. One other explanation for the high heterogeneity could be the different questionnaires used in the separate studies. For both QoL and depressive symptoms, 13 different rating scales were used. For global cognition, six different tests were used.
Implications for clinical practice
Currently, physical exercise is not a standard part of the treatment of the six chronic brain disorders included in this study. Based on our work, it is likely that patients with any of the investigated brain disorders could benefit from additional physical exercise therapy. As safety issues and age constraints do not seem to be a limiting factor, healthcare professionals could use the present findings to provide patients with a tailored intervention in terms of type of exercise, exercise time and duration of intervention period. We showed a positive dose–effect interaction for exercise time, indicating that longer exercise programs are better for mood improvement. Most studies included in our meta-analysis assessed supervised exercise. Therefore, our results cannot be generalized to unsupervised exercise.
Implications for further research
Given the purpose and transdiagnostic character of the present study, we chose to compare exercise intervention only to TAU control condition. Evaluation of any differential effects of other components of the interventions such as adherence, setting (e.g., home-based vs. gym-based), monitoring of exercise sessions with instruments (e.g., heart rate meters), cost-effectiveness and comparison with other control groups (e.g., active control conditions) is required to provide detailed recommendations on physical exercise interventions for the clinical practice.
Strengths and limitations
The greatest strength of the present study is that it provides an up-to-date and extensive quantitative overview of the literature regarding the efficacy of different exercise interventions in patients with chronic brain disorders. Second, our findings are largely in accordance with previous (quantitative) reviews that synthesized evidence on the efficacy of physical exercise in the studied brain disorders [20, 22, 24, 25, 28, 252]. However, in contrast to previous work, we performed both transdiagnostic and within-disorder analyses and evaluated the effect of several moderators providing evidence that physical exercise can be considered as an effective add-on and transdiagnostic treatment.
This study has some limitations. First, several studies could not be included in the cognitive meta-analyses, so that the overall effect of exercise on cognition was based on fewer studies than the other meta-analyses, making these findings more susceptible to change over time (when more studies become available). Notably, a recent RCT of 4-month aerobic and resistance exercise of moderate to high intensity added to usual care found that physical exercise did not slow cognitive decline in patients with mild-to-moderate dementia [18]. The authors measured global cognition with Alzheimer’s disease assessment scale-cognitive subscale (ADAS-cog) and found a small average difference with uncertain clinical relevance. This study did not fulfill the inclusion criteria of our study to be included in the quantitative review. However, considering the fact that we included four RCTs [65, 68, 74, 224] with negative outcomes of exercise on global cognition in AD (see Fig. 4) and did not find a significant overall effect of exercise on global cognition, we do not expect that adding this study would have changed our findings. Second, the analysis regarding the effect of physical exercise on depressive symptoms included studies with different disorders, and the included studies also differed in the severity of depression, ranging from mild depression to the presence of major depressive disorder. This might have biased the findings and resulted in a high effect size. However, both low and high effect sizes were found in mild and major depression, which suggests that physical exercise is effective for depressive symptoms in general, irrespective of the underlying severity. Third, publication bias is an important possible drawback in meta-analytical studies. Egger’s test showed potential publication bias for QoL and depressive symptoms. However, the fail-safe numbers of these tests were extremely large, increasing the validity of the results. Fourth, heterogeneity among studies was high, possibly due to combining studies with largely different interventions offered to different groups. However, heterogeneity values of the joint analysis were lower than the within-disorder heterogeneities (Tables 1, 2), indicating consistency in studies across disorders so that joint analysis of disorders deemed sensible. Moreover, one of the main inter-study differing variables, age, did not affect the efficacy of exercise on the outcome measures. Besides, Q- and I2-statistic cannot be used to estimate the magnitude of true dispersion [253]. Fifth, for all outcome measures, the risk of bias assessment indicated highest risk in terms of attrition. Incomplete outcome data and lack of ITT-analysis in studies could have biased the observed results. However, to account for possible attrition bias, we performed separate analyses on studies that performed ITT-analysis and thus had low risk of bias and studies with unclear risk of bias on ITT analysis (i.e., insufficient information to judge). These results showed even higher effects of exercise on QoL and depressive symptoms, while effects on cognition remained similar for the cognitive domain PS, but turned to non-significance for the cognitive domains A and WM, EF and M. The latter is likely due to the moderate to high heterogeneity among studies after inclusion of the study by [219]. Finally, we randomly selected six brain disorders of various etiology (e.g., neurodegenerative, neurodevelopmental, inflammatory) to demonstrate the generalizability of efficacy of exercise. Since we did not find any RCTs evaluating the effect of physical exercise in bipolar disorder, we decided to only include unipolar depression in the present study. Other brain disorders, such as epilepsy, traumatic brain injury and migraine have been investigated as well, but given restriction in time and capacity (as well as wordcount), this paper was confined to the chronic brain disorders summed above.
Conclusion
Additional therapy with physical exercise in patients with chronic brain disorders seems safe and has a medium-sized effect on QoL and a large beneficial effect on depressive symptoms, with a positive dose–response correlation. The evidence for the efficacy on cognition is small, but clinically relevant. Therefore, to improve the health status of patients with chronic brain disorders, add-on exercise therapy should be considered as an essential part of the treatment.
References
van Uem J, Marinus J, Canning C, van Lummel R, Dodel R, Liepelt-Scarfone I et al (2015) Health-related quality of life in patients with Parkinson’s disease—a systematic review based on the ICF model. Neurosci Biobehav Rev 61:26–34
Berrigan LI, Fisk JD, Patten SB, Tremlett H, Wolfson C, Warren S et al (2016) Health-related quality of life in multiple sclerosis: direct and indirect effects of comorbidity. Neurology
Karow A, Bullinger M, Lambert M (2016) Quality of life as an outcome and a mediator of other outcomes in patients with schizophrenia. Beyond assessment of quality of life in schizophrenia. Springer, Berlin, pp 123–144
Ready RE, Mathews M, Leserman A, Paulsen JS (2008) Patient and caregiver quality of life in Huntington’s disease. Mov Disord Soc 23:721–726
Feinstein A (2011) Multiple sclerosis and depression. Mult Scler 17(11):1276–1281
Pfeiffer RF (2016) Non-motor symptoms in Parkinson’s disease. Park Relat Disord 22:S119–S122
Conde-Sala JL, Turrõ-Garriga O, Piñán-Hernández S, Portellano-Ortiz C, Viñas-Diez V, Gascõn-Bayarri J et al (2016) Effects of anosognosia and neuropsychiatric symptoms on the quality of life of patients with Alzheimer’s disease: a 24-month follow-up study. Int J Geriatr Psychiatry 31(2):109–119
Brissos S, Pereira G, Balanzá-Martinez V (2016) Quality of life, cognition, and social cognition in schizophrenia. Beyond assessment of quality of life in schizophrenia. Springer, Berlin, pp 25–51
Pirogovsky-Turk E, Moore RC, Filoteo JV, Litvan I, Song DD, Lessig SL et al (2016) Neuropsychiatric predictors of cognitive decline in Parkinson disease: a longitudinal study. Am J Geriatr Psychiatry 2016:1–11
Adamson BC, Ensari I, Motl RW (2015) Effect of exercise on depressive symptoms in adults with neurologic disorders: a systematic review and meta-analysis. Arch Phys Med Rehabil 96(7):1329–1338
Fayers PM, Machin D (2013) Quality of life: the assessment, analysis and interpretation of patient-reported outcomes. Wiley, New York
Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC et al (2016) AHA/ASA guideline guidelines for adult stroke rehabilitation and recovery, pp 1–73
Billinger SA, Arena R, Bernhardt J, Eng JJ, Ot PT, Franklin BA et al (2014) AHA/ASA scientific statement stroke survivors
Foundation NS (2010) Clinical guidelines for stroke management 2010
Party SW (2016) National clinical guideline for stroke
Excellence NI for H and C (2016) Strok stroke e in adults. Natl Inst Health Care Excell
Groot C, Hooghiemstra AM, Raijmakers PGHM, van Berckel BNM, Scheltens P, Scherder EJA et al (2016) The effect of physical activity on cognitive function in patients with dementia: a meta-analysis of randomized control trials. Ageing Res Rev 25:13–23
Lamb SE, Sheehan B, Atherton N, Nichols V, Collins H, Mistry D et al (2018) Dementia and physical activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ 361:k1675
Demaneuf T, Aitken Z, Karahalios A, Leong TI, De Livera AM, Jelinek GA et al (2018) The effectiveness of exercise interventions for pain reduction in people with multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil
Campbell E, Coulter EH, Paul L (2018) High intensity interval training for people with multiple sclerosis: a systematic review. Mult Scler Relat Disord 24:55–63
Etoom M, Khraiwesh Y, Lena F, Hawamdeh M, Hawamdeh Z, Centonze D et al (2018) Effectiveness of physiotherapy interventions on spasticity in people with multiple sclerosis. A systematic review and meta-analysis. Am J Phys Med Rehabil
Fayyaz M, Jaffery SS, Anwer F, Zil-E-Ali A, Anjum I (2018) The effect of physical activity in Parkinson’s disease: a mini-review. Cureus 10(7):e2995
da Silva FC, da Iop RR, de Oliveira LC, Boll AM, de Alvarenga JGS, Gutierres Filho PJB et al (2018) Effects of physical exercise programs on cognitive function in Parkinson’s disease patients: a systematic review of randomized controlled trials of the last 10 years. PLoS One 13(2):0193113
Dauwan M, Begemann MJH, Heringa SM, Sommer IE (2015) Exercise improves clinical symptoms, quality of life, global functioning, and depression in schizophrenia: a systematic review and meta-analysis. Schizophr Bull 2015:sbv164
Firth J, Cotter J, Elliott R, French P, Yung AR (2015) A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol Med 45(07):1343–1361
Brondino N, Rocchetti M, Fusar-Poli L, Codrons E, Correale L, Vandoni M et al (2017) A systematic review of cognitive effects of exercise in depression. Acta Psychiatr Scand 135(4):285–295
Sun M, Lanctot K, Herrmann N, Gallagher D (2018) Exercise for cognitive symptoms in depression: a systematic review of interventional studies. Can J Psychiatry 63(2):115–128
Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, Stubbs B (2016) Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res 77:42–51
Lam LCW (2016) Physical exercise interventions for mental health. Cambridge University Press, Cambridge
Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14(4):388–405
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8(4):382–397
Frohman EM, Racke MK, Raine CS (2006) Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med 354(9):942–955
Steiner J, Walter M, Glanz W, Sarnyai Z, Bernstein H-G, Vielhaber S et al (2013) Increased prevalence of diverse N-methyl-d-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia: specific relevance of IgG NR1a antibodies for distinction from N-methyl-d-aspartate glutamate receptor encephalitis. JAMA Psychiatry 70(3):271–278
Lotharius J, Brundin P (2002) Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci 3(12):932–942
Stepnicki P, Kondej M, Kaczor AA (2018) Current concepts and treatments of schizophrenia. Molecules 23:8
Lopez-Figueroa AL, Norton CS, Lopez-Figueroa MO, Armellini-Dodel D, Burke S, Akil H et al (2004) Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol Psychiatry 55(3):225–233
Angelucci F, Brene S, Mathe AA (2005) BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry 10(4):345–352
Parain K, Murer MG, Yan Q, Faucheux B, Agid Y, Hirsch E et al (1999) Reduced expression of brain-derived neurotrophic factor protein in Parkinson’s disease substantia nigra. NeuroReport 10(3):557–561
Bonavita S, Gallo A, Sacco R, Corte MD, Bisecco A, Docimo R et al (2011) Distributed changes in default-mode resting-state connectivity in multiple sclerosis. Mult Scler 17(4):411–422
Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD (2007) Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry 164(3):450–457
Greicius MD, Srivastava G, Reiss AL, Menon V (2004) Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 101(13):4637–4642
Tessitore A, Esposito F, Vitale C, Santangelo G, Amboni M, Russo A et al (2012) Default-ode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology 79(23):2226–2232
Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L et al (2018) Analysis of shared heritability in common disorders of the brain. Science 360:6395
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ Engl 339:2535
Cuijpers P, Li J, Hofmann SG, Andersson G (2010) Self-reported versus clinician-rated symptoms of depression as outcome measures in psychotherapy research on depression: a meta-analysis. Clin Psychol Rev 30(6):768–778
Williams JBW (1988) A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry 45(8):742–747
Beck AT, Steer RA, Brown GK (1996) Beck depression inventory-II. San Antonio 78(2):490–498
Carmody TJ, Rush AJ, Bernstein I, Warden D, Brannan S, Burnham D et al (2006) The Montgomery Äsberg and the Hamilton ratings of depression: a comparison of measures. Eur Neuropsychopharmacol 16(8):601–611
Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M et al (1982) Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 17(1):37–49
Spitzer RL, Kroenke K, Williams JBW, Group PHQPCS (1999) Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. JAMA 282(18):1737–1744
McNair DM, Droppleman LF, Lorr M (1992) Edits manual for the profile of mood states: POMS. Edits, San Diego
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions. Cochrane Collab [Internet]; Version 5. http://www.handbook.cochrane.org
Deeks JJ, Group M, Points K. 16 special topics in statistics. In: JPT Higgins, JJ Deeks (eds)
Borenstein M, Hedges LV, Higgins J, Rothstein HR (2009) Multiple outcomes or time-points within a study. Introduction to meta-analysis. Wiley, Oxford, pp 225–238
Borenstein M, Hedges LV, Higgins J, Rothstein HR (2009) Random-effects model. Introduction to meta-analysis. Wiley, Oxford, pp 69–75
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum, New Jersey
Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J et al (2011) Recommendations for examining and interpreting funnel plot asymmetry in metaanalyses of randomised controlled trials. BMJ 343(7109):d4002
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in metaanalyses. BMJ Br Med J 327:557–560
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(September):629–634
Rosenthal R (1979) The file drawer problem and tolerance for null results. Psychol Bull 86(3):638–641
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM et al (2011) Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43(7):1334–1359
Aguiar P, Monteiro L, Feres A, Gomes I, Melo A (2014) Rivastigmine transdermal patch and physical exercises for Alzheimer’s disease: a randomized clinical trial. Curr Alzheimer Res 11(6):532–537
Arcoverde C, Deslandes A, Moraes H, Almeida C, de Araujo NB, Vasques PE et al (2014) Treinamento na esteira como um tratamento adicional para a doença de Alzheimer: Estudo piloto controlado randomizado. Arq Neuropsiquiatr 72(3):190–196
Hoffmann K, Sobol NA, Frederiksen KS, Beyer N, Vogel A, Vestergaard K et al (2015) Moderate-to-high intensity physical exercise in patients with Alzheimer’s disease: a randomized controlled trial. J Alzheimer’s Dis 50(2):443–453
Holthoff VA, Marschner K, Scharf M, Steding J, Meyer S, Koch R et al (2015) Effects of physical activity training in patients with alzheimer’s dementia: results of a pilot RCT study. PLoS One 10(4):1–12
Kemoun G, Thibaud M, Roumagne N, Carette P, Albinet C, Toussaint L et al (2010) Effects of a physical training programme on cognitive function and walking efficiency in elderly persons with dementia. Dement Geriatr Cogn Disord 29(2):109–114
Lautenschlager NT, Cox KL, Flicker L, Cyarto E, Ames D, Logiudice D et al (2015) A randomized controlled trial evaluating the effects of physical activity in people with 29 Alzheimer’s disease: the fitness for the ageing brain study ii (fabs II). Alzheimer’s Dement 11(7):P280–P281
Maci T, Pira FL, Quattrocchi G, Nuovo SD, Perciavalle V, Zappia M (2012) Physical and cognitive stimulation in Alzheimer disease. The GAIA project: a pilot study. Am J Alzheimers Dis Other Demen 27(2):107–113
Roach KE, Tappen RM, Kirk-Sanchez N, Williams CL, Loewenstein D (2011) A randomized controlled trial of an activity specific exercise program for individuals with Alzheimer disease in long-term care settings. J Geriatr Phys Ther 34(2):50–56
Rolland Y, Pillard F, Klapouszczak A, Reynish E, Thomas D, Andrieu S et al (2007) Exercise program for nursing home residents with Alzheimer’s disease: a 1-year randomized, controlled trial. J Am Geriatr Soc 55(2):158–165
Steinberg M, Leoutsakos JS, Podewils LJ, Lyketsos CG (2009) Evaluation of a home-based exercise program in the treatment of Alzheimer’s disease: the Maximizing Independence in Dementia (MIND) study. Int J Geriatr Psychiatry 24(7):680–685
Suttanon P, Hill KD, Said CM, Williams SB, Byrne KN, LoGiudice D et al (2012) Feasibility, safety and preliminary evidence of the effectiveness of a home-based exercise programme for older people with Alzheimer’s disease: a pilot randomized controlled trial. Clin Rehabil 27(5):427–438
Teri L, Gibbons LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE et al (2003) Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA 290(15):2015–2022
Venturelli M, Scarsini R, Schena F (2011) Six-month walking program changes cognitive 30 and ADL performance in patients with Alzheimer. Am J Alzheimers Dis Other Demen 26(5):381–388
Vreugdenhil A, Cannell J, Davies A, Razay G (2012) A community-based exercise programme to improve functional ability in people with Alzheimer’s disease: a randomized controlled trial. Scand J Caring Sci 26(1):12–19
Ygüez L, Shaw KN, Morris R, Matthews D (2011) The effects on cognitive functions of a movement-based intervention in patients with Alzheimer’s type dementia: a pilot study. Int J Geriatr Psychiatry 26(2):173–181
Yang S-Y, Shan C-L, Qing H, Wang W, Zhu Y, Yin M-M et al (2015) The effects of aerobic exercise on cognitive function of Alzheimer’s disease patients. CNS Neurol Disord Drug Targets 14(10):1292–1297
Zhang Y-H, Lu S-P, Xu Y-N, Fu X, Huang Q (2004) Effect of one-year rehabilitation training in patients with Alzheimer disease. Chin J Clin Rehabil 8(31):6859–6861
Blumenthal J, Babyak M, Moore K (1999) Effects of exercise training on older patients with major depression. Arch Intern Med 159:2349–2356
Blumenthal JA, Babyak MA, Doraiswamy M, Watkins L, Hoffman B, Barbour K et al (2007) Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med 69(13):587–596
Brenes GA, Williamson JD, Messier SP, Rejeski WJ, Pahor M, Ip E et al (2007) Treatment of minor depression in older adults: a pilot study comparing sertraline and exercise. Aging Ment Health 11(1):61–68
Carneiro LSF, Fonseca AM, Vieira-Coelho MA, Mota MP, Vasconcelos-Raposo J (2015) Effects of structured exercise and pharmacotherapy vs. pharmacotherapy for adults with depressive symptoms: a randomized clinical trial. J Psychiatr Res 71:48–55
Carta MG, Hardoy MC, Pilu A, Sorba M, Floris AL, Mannu FA et al (2008) Improving physical quality of life with group physical activity in the adjunctive treatment of major depressive disorder. Clin Pract Epidemiol Ment Health 4(1):1
Carter T, Guo B, Turner D, Morres I, Khalil E, Brighton E et al (2015) Preferred intensity exercise for adolescents receiving treatment for depression: a pragmatic randomised controlled trial. BMC Psychiatry 15:1–12
Siqueira CC, Valiengo LL, Carvalho AF, Santos-Silva PR, Missio G, De Sousa RT et al (2016) Antidepressant efficacy of adjunctive aerobic activity and associated biomarkers in major depression: a 4-week, randomized, single-blind, controlled clinical trial. PLoS One 11(5):1–12
Chan AS, Wong QY, Sze SL, Kwong PPK, Han YMY, Cheung MC (2012) A Chinese Chanbased mind-body intervention for patients with depression. J Affect Disord 142(1–3):283–289
Chou KL, Lee PWH, Yu ECS, Macfarlane D, Cheng YH, Chan SSC et al (2004) Effect of Tai Chi on depressive symptoms amongst Chinese older patients with depressive disorders: a randomized clinical trial. Int J Geriatr Psychiatry 19(11):1105–1107
Danielsson L, Papoulias I, Petersson EL, Carlsson J, Waern M (2014) Exercise or basic body awareness therapy as add-on treatment for major depression: a controlled study. J Affect Disord 168:98–106
Doose M, Ziegenbein M, Hoos O, Reim D, Stengert W, Hoffer N et al (2015) Self-selected intensity exercise in the treatment of major depression: a pragmatic RCT. Int J Psychiatry Clin Pract 19(4):266–276
Hoffman BM, Blumenthal JA, Babyak MA, Smith PJ, Rogers SD, Doraiswamy PM et al (2008) Exercise fails to improve neurocognition in depressed middle-aged and older adults. Med Sci Sports Exerc 40(7):1344–1352
Huang T-T, Liu C-B, Tsai Y-H, Chin Y-F, Wong C-H (2015) Physical fitness exercise vs. cognitive behavior therapy on reducing the depressive symptoms among community dwelling elderly adults: a randomized controlled trial. Int J Nurs Stud 52(10):1542–1552
Kerling A, Tegtbur U, Gützlaff E, Kück M, Borchert L, Ates Z et al (2015) Effects of adjunctive exercise on physiological and psychological parameters in depression: a randomized pilot trial. J Affect Disord 177:1–6
Kerse N, Hayman KJ, Moyes SA, Peri K, Robinson E, Dowell A et al (2010) Home-based activity program for older people with depressive symptoms: DeLLITE—a randomized controlled trial. Ann Fam Med 8(3):214–223
Khatri P, Blumenthal JA, Babyak MA, Craighead EW, Herman S, Baldewicz T et al (2001) Effects of exercise training on cognitive functioning among depressed older men and women. J Aging Phys Activ 9:43–57
Kinser PA, Elswick RK, Kornstein S (2014) Potential long-term effects of a mind-body intervention for women with major depressive disorder: sustained mental health improvements with a pilot yoga intervention. Arch Psychiatr Nurs 28(6):377–383
Lavretsky H, Alstein LL, Olmstead RE, Ercoli LM, Riparetti-Brown M, St. Cyr N et al (2011) Complementary use of Tai Chi Chih augments escitalopram treatment of geriatric depression: a randomized controlled trial. Am J Geriatr Psychiatry 19(10):839–850
Legrand FD (2015) Effects of exercise on physical self-concept, global self-esteem, and depression in women of low socioeconomic status with elevated depressive symptoms. J Sport Exerc Psychol 1:357–365
Legrand FD, Neff EM (2016) Efficacy of exercise as an adjunct treatment for clinically depressed inpatients during the initial stages of antidepressant pharmacotherapy: an open randomized controlled trial. J Affect Disord 191:139–144
Luttenberger K, Stelzer E-M, Först S, Schopper M, Kornhuber J, Book S (2015) Indoor rock climbing (bouldering) as a new treatment for depression: study design of a waitlist controlled randomized group pilot study and the first results. BMC Psychiatry 15(1):201
Mather AS, Rodriguez C, Guthrie MF, McHarg AM, Reid IC, McMurdo MET (2002) Effects of exercise on depressive symptoms in older adults with poorly responsive depressive disorder: randomised controlled trial. Br J Psychiatry 180:411–415
Mota-Pereira J, Silverio J, Carvalho S, Ribeiro JC, Fonte D, Ramos J (2011) Moderate exercise improves depression parameters in treatment-resistant patients with major depressive disorder. J Psychiatr Res 45(8):1005–1011
Belvederi Murri M, Amore M, Menchetti M, Toni G, Neviani F, Cerri M et al (2015) Physical exercise for late-life major depression. Br J Psychiatry 207(3):235–242
Nabkasorn C, Miyai N, Sootmongkol A, Junprasert S, Yamamoto H, Arita M et al (2006) Effects of physical exercise on depression, neuroendocrine stress hormones and physiological fitness in adolescent females with depressive symptoms. Eur J Public Health 16(2):179–184
Niemi M, Kiel S, Allebeck P, Hoan LT (2016) Community-based intervention for depression management at the primary care level in Ha Nam Province, Vietnam: a cluster randomised controlled trial. Trop Med Int Health 21(5):654–661
Oertel-Knöchel V, Mehler P, Thiel C, Steinbrecher K, Malchow B, Tesky V et al (2014) Effects of aerobic exercise on cognitive performance and individual psychopathology in depressive and schizophrenia patients. Eur Arch Psychiatry Clin Neurosci 1:1–16
Pfaff JJ, Alfonso H, Newton RU, Sim M, Flicker L, Almeida OP (2014) ACTIVEDEP: a randomised, controlled trial of a home-based exercise intervention to alleviate depression in middle-aged and older adults. Br J Sports Med 48(3):226–232
Pilu A, Sorba M, Hardoy MC, Floris AL, Mannu F, Seruis ML et al (2007) Efficacy of physical activity in the adjunctive treatment of major depressive disorders: preliminary results. Clin Pract Epidemiol Ment Health 3(1):1
Prakhinkit S, Suppapitiporn S, Tanaka H, Suksom D (2014) Effects of Buddhism Walking Meditation on depression, functional fitness, and endothelium-dependent vasodilation in depressed elderly. J Altern Complement Med 20(5):411–416
Schuch FB, Vasconcelos-Moreno MP, Borowsky C, Zimmermann AB, Rocha NS, Fleck MP (2015) Exercise and severe major depression: effect on symptom severity and quality of life at discharge in an inpatient cohort. J Psychiatr Res 61:25–32
Shahidi M, Mojtahed A, Modabbernia A, Mojtahed M, Shafiabady A, Delavar A et al (2011) Laughter yoga versus group exercise program in elderly depressed women: a randomized controlled trial. Int J Geriatr Psychiatry 26(3):322–327
Sims J, Hill K, Davidson S, Gunn J, Huang N (2006) Exploring the feasibility of a community-based strength training program for older people with depressive symptoms and its impact on depressive symptoms. BMC Geriatr 6:18
Singh NA, Clements KM, Fiatarone MA (1997) A randomized controlled trial of progressive resistance training in depressed elders. J Gerontol Ser A Biol Sci Med Sci 52(1):M27–M35
Singh NA, Clements KM, Singh MA (2001) The efficacy of exercise as a long-term antidepressant in elderly subjects: a randomized, controlled trial. J Gerontol 56(8):M497–M504
Singh NA, Stavrinos TA, Scarbek Y, Galambos G, Liber C, Singh MAF (2005) A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. J Gerontol Ser A Biol Sci Med Sci 60(6):768–776
Tsang HWH, Fung KMT, Chan ASM, Lee G, Chan F (2006) Effect of a qigong exercise programme on elderly with depression. Int J Geriatr Psychiatry 21(9):890–897
Tsang HWH, Tsang WWN, Jones AYM, Fung KMT, Chan AHL, Chan EP et al (2012) Psycho-physical and neurophysiological effects of qigong on depressed elders with chronic illness. Aging Ment Health 17(3):1–13
Veale D, Le Fevre K, Pantelis C, de Souza V, Mann A, Sargeant A (1992) Aerobic exercise in the adjunctive treatment of depression: a randomized controlled trial. J R Soc Med 85(9):541–544
Yeung A, Lepoutre V, Wayne P, Yeh G, Slipp LE, Fava M et al (2012) Tai chi treatment for depression in Chinese Americans: a pilot study. Am J Phys Med Rehabil 91(10):863–870
Busse M, Quinn L, Debono K, Jones K, Collett J, Playle R et al (2013) A randomized feasibility study of a 12-week community-based exercise program for people with Huntington’s disease. J Neurol Phys Ther 37(4):149–158
Khalil H, Quinn L, van Deursen R, Dawes H, Playle R, Rosser A et al (2013) What effect does a structured home-based exercise programme have on people with Huntington’s disease? A randomized, controlled pilot study. Clin Rehabil 27(7):646–658
Quinn L, Debono K, Dawes H, Rosser AE, Nemeth AH, Rickards H et al (2014) Taskspecific training in huntington disease: a randomized controlled feasibility trial. Phys Ther 94:1555
Thompson JA, Cruickshank TM, Penailillo LE, Lee JW, Newton RU, Barker RA et al (2013) The effects of multidisciplinary rehabilitation in patients with early-to-middle-stage Huntington’s disease: a pilot study. Eur J Neurol 20(9):1325–1329
Ahmadi A, Nikbakh M, Arastoo AA, Habibi A-H (2010) The effects of a yoga intervention on balance, speed and endurance of walking, fatigue and quality of life in people with multiple sclerosis. J Hum Kinet 23(January):71–78
Ahmadi A, Arastoo AA, Nikbakht M (2010) The effects of a treadmill training programme on balance, speed and endurance walking, fatigue and quality of life in people with multiple sclerosis. Int Sport J 11:4
Ahmadi A, Arastoo AA, Nikbakht M, Zahednejad S, Rajabpour M (2013) Comparison of the effect of 8 weeks aerobic and yoga training on ambulatory function, fatigue and mood status in MS patients. Iran Red Crescent Med J 15(6):449–454
Bernhardt L, Jolk C, Alcantara R, Platen P, Marziniak M, Weßling K (2012) The effects of resistance training and physical activities in groups in comparison for the treatment of chronic fatigue in patients with multiple sclerosis. Mult Scler 18(4):247
Bjarnadottir OH, Konradsdottir AD, Reynisdottir K, Olafsson E (2007) Multiple sclerosis and brief moderate exercise. A randomised study. Mult Scler 13(6):776–782
Briken S, Gold SM, Patra S, Vettorazzi E, Harbs D, Tallner A et al (2014) Effects of exercise on fitness and cognition in progressive MS: a randomized, controlled pilot trial. Mult Scler 20(3):382–390
Bulguroglu I, Guclu-Gunduz A, Gokhan Y, Ozkul C, Irkec C, Batur-Caglayan HZ et al (2015) Comparison of the effects of mat Pilates and reformer Pilates on balance, strength, mobility, fatique and quality of life in patients with multiple sclerosis. Eur J Neurol 22:672
Çakt BD, Nacir B, Genç H, Saraçoǧlu M, Karagöz A, Erdem HR et al (2010) Cycling progressive resistance training for people with multiple sclerosis: a randomized controlled study. Am J Phys Med Rehabil 89(6):446–457
Carter A, Daley A, Humphreys L, Snowdon N, Woodroofe N, Petty J et al (2014) Pragmatic intervention for increasing self-directed exercise behaviour and improving important health outcomes in people with multiple sclerosis: a randomised controlled trial. Mult Scler 20(8):1112–1122
Dalgas U, Stenager E, Jakobsen J, Petersen T, Hansen HJ, Knudsen C et al (2010) Fatigue, mood and quality of life improve in MS patients after progressive resistance training. Mult Scler 16(4):480–490
Dodd KJ, Taylor NF, Shields N, Prasad D, McDonald E, Gillon A (2011) Progressive resistance training did not improve walking but can improve muscle performance quality of life and fatigue in adults with multiple sclerosis: a randomized controlled trial. Mult Scler 17(11):1362–1374
Doulatabad N, Tradit AJ, Altern C (2013) Afr J Tradit Complement Altern Med 2013(10):49–52
Ebrahimi A, Eftekhari E, Etemadifar M (2015) Effects of whole body vibration on hormonal and functional indices in patients with multiple sclerosis. Indian J Med Res 142(OCTOBER):450–458
Feys P, Moumdjian L, Vanhalewyck F, Wens I, Op ’t Eijnde B, Van Wijmeersch B et al (2016) Effects of an individual 12 weeks community located running program on physical capacity, walking, cognitive function, dual tasking and brain volumes and structures in persons with multiple sclerosis. Mult Scler 22:73–74
Garrett M, Hogan N, Larkin A, Saunders J, Jakeman P, Coote S (2012) Exercise in the community for people with minimal gait impairment due to MS: an assessor-blind randomized controlled trial. Mult Scler J 19:1352458512461966
Hebert JR, Corboy JR, Manago MM, Schenkman M (2012) Effects of vestibular rehabilitation on multiple sclerosis-related fatigue and upright postural control: a randomized controlled trial. In: 137th annual meeting american neurological association, Boston, MA, USA [Internet], vol 72, pp S34–S35
Hoang P, Schoene D, Gandevia S, Smith S, Lord SR (2015) Effects of a home-based step training programme on balance, stepping, cognition and functional performance in people with multiple sclerosis—a randomized controlled trial. Mult Scler 22:1–10
Hogan N, Kehoe M, Larkin A, Coote S (2014) The effect of community exercise interventions for people with MS who use bilateral support for gait. Mult Scler Int 2014:109142
Jäckel N, Tallner A, Virsevci Ö, Denkinger N, Sebald K, Mäurer M et al (2015) Effects of internet-based exercise (e-training) on fatigue and other patient reported behavioural outcomes (PRO) in patients with relapsing-remitting multiple sclerosis (RRMS). Mult Scler 23(11):616
Kargarfard M, Etemadifar M, Baker P, Mehrabi M, Hayatbakhsh R (2012) Effect of aquatic exercise training on fatigue and health-related quality of life in patients with multiple sclerosis. Arch Phys Med Rehabil 93(10):1701–1708
Khan F, Pallant JF, Brand C, Kilpatrick TJ (2008) Effectiveness of rehabilitation intervention in persons with multiple sclerosis: a randomised controlled trial. J Neurol Neurosurg Psychiatry 79(11):1230–1235
Kooshiar H, Moshtagh M, Sardar MA, Foroughipour M, Shakeri MT, Vahdatinia B (2015) Fatigue and quality of life of women with multiple sclerosis: a randomized controlled clinical trial. J Sports Med Phys Fitness 55(6):668–674
Learmonth YC, Paul L, Miller L, Mattison P, McFadyen AK (2012) The effects of a 12-week leisure centre-based, group exercise intervention for people moderately affected with multiple sclerosis: a randomized controlled pilot study. Clin Rehabil 26(7):579–593
Louie J, Baquie KA, Offerman J, Bower KJ, Granger CL, Khan F (2015) Maximising abilities, negotiating and generating exercise options (manage) program: a pilot randomised controlled trial in persons with multiple sclerosis. Physiother (United Kingdom) 101:eS901–eS902
McCullagh R, Fitzgerald AP, Murphy RP, Cooke G (2008) Long-term benefits of exercising on quality of life and fatigue in multiple sclerosis patients with mild disability: a pilot study. Clin Rehabil 22(3):206–214
Miller L, Paul L, Mattison P, McFadyen A (2011) Evaluation of a home-based physiotherapy programme for those with moderate to severe multiple sclerosis: a randomized controlled pilot study. Clin Rehabil 25(8):720–730
Negahban H, Rezaie S, Goharpey S (2013) Massage therapy and exercise therapy in patients with multiple sclerosis: a randomized controlled pilot study. Clin Rehabil 27(12):1126–1136
Nilsagård YE, Forsberg AS, von Koch L (2013) Balance exercise for persons with multiple sclerosis using Wii games: a randomised, controlled multi-centre study. Mult Scler 19(2):209–216
Donnell M, Coote S (2011) Physiotherapy intervention in persons with MS who are nonambulatory. Physiother (United Kingdom) 97:eS917
Oken BS, Kishiyama S, Zajdel D, Bourdette D, Carlsen J, Haas M et al (2004) Randomized controlled trial of yoga and exercise in multiple sclerosis. Neurology 62(11):2058–2064
Ozgen G (2016) Is customized vestibular rehabilitation effective in patients with multiple sclerosis? A randomized controlled trial. Eur J Phys Med Rehabil 52:1–29
Paul L, Coulter EH, Miller L, McFadyen A, Dorfman J, Mattison PGG (2014) Web-based physiotherapy for people moderately affected with Multiple Sclerosis; quantitative and qualitative data from a randomized, controlled pilot study. Clin Rehabil 28(9):924–935
Petajan JH, Gappmaier E, White AT, Spencer MK, Mino L, Hicks RW (1996) Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol 39(4):432–441
Plow M, Bethoux F, Mai K, Marcus B (2014) A formative evaluation of customized pamphlets to promote physical activity and symptom self-management in women with multiple sclerosis. Health Educ Res 29(5):883–896
Prosperini L, Fortuna D, Gianni C, Leonardi L, Marchetti MR, Pozzilli C (2013) Home-based balance training using the Wii balance board: a randomized, crossover pilot study in multiple sclerosis. Neurorehabil Neural Repair 27(6):516–525
Rahnama N, Namazizadeh M, Etemadifar M, Bambaeichi E, Arbabzadeh S, Sadeghipour HR (2011) Effects of yoga on depression in women with multiple sclerosis. J Isfahan Med Sch 29:136
Razazian N, Yavari Z, Farnia V, Azizi A, Kordavani L, Bahmani DS et al (2016) Exercising impacts on fatigue, depression, and paresthesia in female patients with multiple sclerosis. Med Sci Sports Exerc 48(5):796–803
Rietberg MB, Van Wegen EEH, Eyssen ICJM, Kwakkel G (2014) Effects of multidisciplinary rehabilitation on chronic fatigue in multiple sclerosis: a randomized controlled trial. PLoS One 9:9
Romberg A, Virtanen A, Ruutiainen J (2005) Long-term exercise improves functional impairment but not quality of life in multiple sclerosis. J Neurol 252(7):839–845
Salhofer-Polanyi S, Windt J, Sumper H, Grill H, Essmeister M, Diermayr G et al (2013) Benefits of inpatient multidisciplinary rehabilitation in multiple sclerosis. NeuroRehabilitation 33(2):285–292
Sandroff BM, Balto JM, Klaren RE, Sommer SK, DeLuca J, Motl RW (2016) Systematically developed pilot randomized controlled trial of exercise and cognition in persons with multiple sclerosis. Neurocase 00(00):1–8
Sangelaji B, Nabavi SM, Estebsari F, Banshi MR, Rashidian H, Jamshidi E et al (2014) Effect of combination exercise therapy on walking distance, postural balance, fatigue and quality of life in multiple sclerosis patients: a clinical trial study. Iran Red Crescent Med J 16(6):e17173
Storr LK, Sorensen PS, Ravnborg M (2006) The efficacy of multidisciplinary rehabilitation in stable multiple sclerosis patients. Mult Scler 12(2):235–242
Straudi S, Martinuzzi C, Pavarelli C, Sabbagh Charabati A, Benedetti MG, Foti C et al (2014) A task-oriented circuit training in multiple sclerosis: a feasibility study. BMC Neurol 14(1):124
Sutherland G, Andersen MB, Stoové MA (2001) Can aerobic exercise training affect health related quality of life for people with multiple sclerosis? J Sport Exerc Psychol 23(2):122–135
Tallner A, Maeurer M, Pfeifer K (2012) An internet-based at home training protocol enhances muscle strength and lung function in multiple sclerosis patients (#133). Mult Scler 18(5):S23–S24
Tarakci E, Yeldan I, Huseyinsinoglu BE, Zenginler Y, Eraksoy M (2013) Group exercise training for balance, functional status, spasticity, fatigue and quality of life in multiple sclerosis: a randomized controlled trial. Clin Rehabil 27(9):813–822
Allen NE, Canning CG, Sherrington C, Lord SR, Latt MD, Close JCT et al (2010) The effects of an exercise program on fall risk factors in people with Parkinson’s disease: a randomized controlled trial. Mov Disord 25(9):1217–1225
Ashburn A, Fazakarley L, Ballinger C, Pickering R, McLellan LD, Fitton C (2007) A randomised controlled trial of a home based exercise programme to reduce the risk of falling among people with Parkinson’s disease. J Neurol Neurosurg Psychiatry 78(7):678–684
Belton A (2014) The effect of a balance exercise class on activity limitations in people with parkinson’s disease
Canning CG, Allen NE, Dean CM, Goh L, Fung VS (2012) Home-based treadmill training for individuals with Parkinson’s disease: a randomized controlled pilot trial. Clin Rehabil 26(9):817–826
Canning CG, Sherrington C, Lord SR, Close JCT, Heller GZ, Howard K et al (2014) Exercise for falls prevention in Parkinson disease: a randomized controlled trial. Am Acad Neurol 84:304–312
Cholewa J, Boczarska-Jedynak M, Opala G (2013) Wpływ systematycznie prowadzonej fizjoterapii na nasilenie objawów ruchowych oraz jakość życia u osób z choroba{ogonek} Parkinsona. Neurol Neurochir Pol 47(3):256–262
Clarke CE, Patel S, Ives N, Rick CE, Dowling F, Woolley R et al (2016) Physiotherapy and occupational therapy vs no therapy in mild to moderate Parkinson disease: a randomized clinical trial. JAMA Neurol 73(3):291–299
Comelia CL, Stebbins GT, Brown-Toms N, Goetz CG (1994) Physical therapy and Parkinson’s disease: a controlled clinical trial. Neurology 44(3 Part 1):376
Conradsson D, Löfgren N, Nero H, Hagströmer M, Ståhle A, Lökk J et al (2015) The effects of highly challenging balance training in elderly with Parkinson’s disease: a randomized controlled trial. Neurorehabil Neural Repair 29(9):827–836
Cugusi L, Solla P, Serpe R, Carzedda T, Piras L, Oggianu M et al (2015) Effects of a Nordic Walking program on motor and non-motor symptoms, functional performance and body composition in patients with Parkinson’s disease. NeuroRehabilitation 37(2):245–254
de Oliveira RT, Felippe LA, Bucken Gobbi LT, Barbieri FA, Christofoletti G (2016) Benefits of exercise on the executive functions in people with Parkinson disease. Am J Phys Med Rehabil 00(00):1
Duncan RP, Earhart GM (2014) Are the effects of community-based dance on Parkinson disease severity, balance, and functional mobility reduced with time? A 2-year prospective pilot study. J Altern Complement Med 20(10):757–763
Foster ER, Golden L, Duncan RP, Earhart GM (2013) Community-based argentine tango dance program is associated with increased activity participation among individuals with parkinson’s disease. Arch Phys Med Rehabil 94(2):240–249
Goodwin VA, Richards SH, Henley W, Ewings P, Taylor AH, Campbell JL (2011) An exercise intervention to prevent falls in people with Parkinson’s disease: a pragmatic randomised controlled trial. J Neurol Neurosurg Psychiatry 82(11):1232–1238
Keus SHJ, Bloem BR, van Hilten JJ, Ashburn A, Munneke M (2007) Effectiveness of physiotherapy in Parkinson’s disease: the feasibility of a randomised controlled trial. Park Relat Disord 13(2):115–121
Laupheimer M, Härtel S, Schmidt S, Bös K (2011) Exercise training—effects of MOTOmed® exercise on typical motor dysfunction in Parkinson s disease. Neurol Rehabil 17(5–6):239–246
Liao Y-Y, Yang Y-R, Cheng S-J, Wu Y-R, Fuh J-L, Wang R-Y (2015) Virtual reality-based training to improve obstacle-crossing performance and dynamic balance in patients with Parkinson’s disease. Neurorehabil Neural Repair 29(7):658–667
Ni M, Mooney K, Signorile JF (2016) Controlled pilot study of the effects of power yoga in Parkinson’s disease. Complement Ther Med 25:126–131
Ni M, Signorile JF, Balachandran A, Potiaumpai M (2016) Power training induced change in bradykinesia and muscle power in Parkinson’s disease. Park Relat Disord 23:37–44
Park A, Zid D, Russell J, Malone A, Rendon A, Wehr A et al (2014) Effects of a formal exercise program on Parkinson’s disease: a pilot study using a delayed start design. Park Relat Disord 20(1):106–111
Picelli A, Varalta V, Melotti C, Zatezalo V, Fonte C, Amato S et al (2016) Effects of treadmill training on cognitive and motor features of patients with mild to moderate Parkinson’s disease: a pilot, single-blind, randomized controlled trial. Funct Neurol 31(1):25–31
Poliakoff E, Galpin AJ, Mcdonald K, Kellett M, Dick JPR, Hayes S et al (2013) The effect of gym training on multiple outcomes in Parkinson’s disease: a pilot randomised waiting-list controlled trial. NeuroRehabilitation 32(1):125–134
Qutubuddin A, Reis T, Alramadhani R, Cifu DX, Towne A, Carne W (2013) Parkinson’s disease and forced exercise: a preliminary study. Rehabil Res Pract 2013:375267
Romenets SR, Anang J, Fereshtehnejad S-M, Pelletier A, Postuma R (2015) Tango for treatment of motor and non-motor manifestations in Parkinson’s disease: a randomized control study. Complement Ther Med 23(2):175–184
Schmitz-Hübsch T, Pyfer D, Kielwein K, Fimmers R, Klockgether T, Wüllner U (2006) Qigong exercise for the symptoms of Parkinson’s disease: a randomized, controlled pilot study. Mov Disord 21(4):543–548
Sharma NK, Robbins K, Wagner K, Colgrove YM (2015) A randomized controlled pilot study of the therapeutic effects of yoga in people with Parkinson’s disease. Int J Yoga 8(1):74
Silva-Batista C, Corcos DM, Roschel H, Kanegusuku H, Gobbi LTB, Piemonte MEP et al (2016) Resistance training with instability for patients with Parkinson’s disease. Med Sci Sports Exerc 48(9):1678–1687
Stack E, Roberts H, Ashburn A (2012) The PIT: SToPP trial—a feasibility randomised controlled trial of home-based physiotherapy for people with Parkinson’s disease using video-based measures to preserve assessor blinding. Parkinsons Dis
Tickle-Degnen L, Ellis T, Saint-Hilaire MH, Thomas CA, Wagenaar RC (2010) Selfmanagement rehabilitation and health-related quality of life in Parkinson’s disease: a randomized controlled trial. Mov Disord 25(2):194–204
Wade DT, Gage H, Owen C, Trend P, Grossmith C, Kaye J (2003) Multidisciplinary rehabilitation for people with Parkinson’s disease: a randomised controlled study. J Neurol Neurosurg Psychiatry 74(2):158–162
Battaglia G, Alesi M, Inguglia M, Roccella M, Caramazza G, Bellafiore M et al (2013) Soccer practice as an add-on treatment in the management of individuals with a diagnosis of schizophrenia. Neuropsychiatr Dis Treat 9:595–603
Ho RTH, Fong TCT, Wan AHY, Au-Yeung FSW, Wong CPK, Ng WYH et al (2016) A randomized controlled trial on the psychophysiological effects of physical exercise and Tai-chi in patients with chronic schizophrenia. Schizophr Res 171(1–3):42–49
Ikai S, Uchida H, Suzuki T, Tsunoda K, Mimura M, Fujii Y (2013) Effects of yoga therapy on postural stability in patients with schizophrenia-spectrum disorders: a single-blind randomized controlled trial. J Psychiatr Res 47(11):1744–1750
Kaltsatou a., Kouidi E, Fountoulakis K, Sipka C, Theochari V, Kandylis D, et al (2014) Effects of exercise training with traditional dancing on functional capacity and quality of life in patients with schizophrenia: a randomized controlled study. Clin Rehabil
Kimhy D, Vakhrusheva J, Bartels MN, Armstrong HF, Ballon JS, Khan S et al (2015) The impact of aerobic exercise on brain-derived neurotrophic factor and neurocognition in individuals with schizophrenia: a single-blind, randomized clinical trial. Schizophr Bull 41(4):859–868
Lin J, Chan SKW, Lee EHM, Chang WC, Tse M, Su WW et al (2015) Aerobic exercise and yoga improve neurocognitive function in women with early psychosis. Nat Publ Gr 1:1–7
Loh SY, Abdullah A, Abu Bakar AK, Thambu M, Nik Jaafar NR (2016) Structured walking and chronic institutionalized schizophrenia inmates: a pilot RCT study on quality of life. Glob J Health Sci 8(1):238–248
Marzolini S, Jensen B, Melville P (2009) Feasibility and effects of a group-based resistance and aerobic exercise program for individuals with severe schizophrenia: a multidisciplinary approach. Ment Health Phys Act 2(1):29–36
Visceglia E, Lewis S (2011) Yoga therapy as an adjunctive treatment for schizophrenia: a randomized, controlled pilot study. J Altern Complement Med 17(7):601–607
Busse M, Quinn L, Drew C, Kelson M, Trubey R, McEwan K et al (2017) Physical activity self-management and coaching compared to social interaction in huntington disease: results from the ENGAGE-HD randomized, controlled pilot feasibility trial. Phys Ther 97(6):625–639
Quinn L, Hamana K, Kelson M, Dawes H, Collett J, Townson J et al (2016) A randomized, controlled trial of a multi-modal exercise intervention in Huntington’s disease. Park Relat Disord 31:46–52
Coghe G, Corona F, Marongiu E, Fenu G, Frau J, Lorefice L et al (2018) Fatigue, as measured using the Modified Fatigue Impact Scale, is a predictor of processing speed improvement induced by exercise in patients with multiple sclerosis: data from a randomized controlled trial. J Neurol 2018:1–6
Duff WRD, Andrushko JW, Renshaw DW, Chilibeck PD, Farthing JP, Danielson J et al (2018) Impact of pilates exercise in multiple sclerosis: a randomized controlled trial. Int J MS Care 20(2):92–100
Learmonth YC, Adamson BC, Kinnett-Hopkins D, Bohri M, Motl RW (2017) Results of a feasibility randomised controlled study of the guidelines for exercise in multiple sclerosis project. Contemp Clin Trials 54:84–97
Vermöhlen V, Schiller P, Schickendantz S, Drache M, Hussack S, Gerber-Grote A et al (2017) Hippotherapy for patients with multiple sclerosis: a multicenter randomized controlled trial (MS-HIPPO). Mult Scler J 2017:1375–1382
Sandroff BM, Johnson CL, Motl RW (2017) Exercise training effects on memory and hippocampal viscoelasticity in multiple sclerosis: a novel application of magnetic resonance elastography. Neuroradiology 59(1):61–67
Carroll LM, Volpe D, Morris ME, Saunders J, Clifford AM (2017) Aquatic exercise therapy for people with parkinson disease: a randomized controlled trial. Arch Phys Med Rehabil 98(4):631–638
Santos L, Fernandez-Rio J, Winge K, Barragán-Pérez B, González-Gómez L, Rodríguez-Pérez V et al (2017) Effects of progressive resistance exercise in akinetic-rigid Parkinson’s disease patients: a randomized controlled trial. Eur J Phys Rehabil Med 53(5):651–663
Vergara-Diaz G, Osypiuk K, Hausdorff JM, Bonato P, Gow BJ, Miranda JG et al (2018) Tai Chi for reducing dual-task gait variability, a potential mediator of fall risk in Parkinson’s disease: a pilot randomized controlled trial. Glob Adv Health Med 7:216495611877538
Bhatia T, Mazumdar S, Wood J, He F, Gur RE, Gur RC et al (2017) A randomised controlled trial of adjunctive yoga and adjunctive physical exercise training for cognitive dysfunction in schizophrenia. Acta Neuropsychiatr 29(2):102–114
Prathikanti S, Rivera R, Cochran A, Tungol JG, Fayazmanesh N, Weinmann E (2017) Treating major depression with yoga: a prospective, randomized, controlled pilot trial. PLoS One 12(3):e0173869
Sharma A, Barrett MS, Cucchiara AJ, Gooneratne NS, Thase ME (2017) A breathing-based meditation intervention for patients with major depressive disorder following inadequate response to antidepressants: a randomized pilot study. J Clin Psychiatry 78(1):e59–e63
Tolahunase MR, Sagar R, Faiq M, Dada R (2018) Yoga- and meditation-based lifestyle intervention increases neuroplasticity and reduces severity of major depressive disorder: a randomized controlled trial. Restor Neurol Neurosci 36(3):423–442
Yeung AS, Feng R, Kim DJH, Wayne PM, Yeh GY, Baer L et al (2017) A pilot, randomized controlled study of Tai Chi with passive and active controls in the treatment of depressed Chinese Americans. J Clin Psychiatry 78(5):e522–e528
Ohman H, Savikko N, Strandberg TE, Kautiainen H, Raivio MM, Laakkonen M-L et al (2016) Effects of exercise on cognition: the Finnish Alzheimer disease exercise trial: a randomized, controlled trial. J Am Geriatr Soc 64(4):731–738
Oertel-Knöchel V, Mehler P, Thiel C, Steinbrecher K, Malchow B, Tesky V et al (2014) Effects of aerobic exercise on cognitive performance and individual psychopathology in depressive and schizophrenia patients. Eur Arch Psychiatry Clin Neurosci 264(7):589–604
Kalron A, Zeilig G (2015) Efficacy of exercise intervention programs on cognition in people suffering from multiple sclerosis, stroke and Parkinson’s disease: a systematic review and meta-analysis of current evidence. NeuroRehabilitation 37(2):273–289
Hindle JV, Petrelli A, Clare L, Kalbe E (2013) Nonpharmacological enhancement of cognitive function in Parkinson’s disease: a systematic review. Mov Disord 28(8):1034–1049
Ranjbar E, Memari AH, Hafizi S, Shayestehfar M, Mirfazeli FS, Eshghi MA (2015) Depression and exercise: a clinical review and management guideline. Asian J Sports Med 6(2):e24055
Netz Y (2017) Is the Comparison between exercise and pharmacologic treatment of depression in the clinical practice guideline of the American college of physicians evidence-based? Front Pharmacol 8(MAY):1–9
NICE (2014) Multiple sclerosis in adults: management. NICE Clin Guidel CG186 2014:20–22
California Department of Public Health (2008) Guideline for Alzheimer’s disease management guideline for Alzheimer’s disease management California Workgroup on Guidelines for Alzheimer’s disease management. Calif Workgr Guidel ALzheimer’s Dis Manag 1:1–122
Galletly C, Castle D, Dark F, Humberstone V, Jablensky A, Killackey E et al (2015) Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Austral N Z J Psychiatry 50:410–472
Keus S, Munneke M, Graziano M, Paltamaa J, Pelosin E, Domingos J et al (2014) European physiotherapy guideline for Parkinson’s disease development and scientific justification. Dev Sci Justif 2014:1–29
Vidal-Jordana A, Montalban X (2017) Multiple sclerosis. Neuroimaging Clin N Am 27(2):195–204
National Collaborating Centre for Mental Health (2014) Psychosis and schizophrenia in adults: treatment and management. Nice. Feb 54 Clinical Guidelines no. 178
Kok RM, Reynolds CF et al (2017) Management of depression in older adults. JAMA 317(20):2114
Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R (2008) Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med 358(3):252–260
Dunkel P, Chai CL, Sperlágh B, Huleatt PB, Mátyus P (2012) Clinical utility of neuroprotective agents in neurodegenerative diseases: current status of drug development for Alzheimer’s, Parkinson’s and Huntington’s diseases, and amyotrophic lateral sclerosis. Expert Opin Investig Drugs 21(9):1267–1308
Huntley JD, Gould RL, Liu K, Smith M, Howard RJ (2015) Do cognitive interventions improve general cognition in dementia? A meta-analysis and meta-regression. BMJ Open 5(4):e005247
Leung I, Walton C, Hallock H, Lewis S, Valenzuela M et al (2015) Cognitive training in Parkinson disease: a systematic review and meta-analysis. Neurology 85(21):1843–1851
Revell ER, Neill JC, Harte M, Khan Z, Drake RJ (2015) A systematic review and metaanalysis of cognitive remediation in early schizophrenia. Schizophr Res 168(1–2):213–222
Rilo O, Peña J, Ojeda N, Rodríguez-Antigüedad A, Mendibe-Bilbao M, Gómez-Gastiasoro A et al (2016) Integrative group-based cognitive rehabilitation efficacy in multiple sclerosis: a randomized clinical trial. Disabil Rehabil 8288:1–9
Clare L, Woods B (2013) Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer’s disease and vascular dementia (review) 6
Nair R, Kj M, Nb L (2016) Memory rehabilitation for people with multiple sclerosis (review) 3
Ströhle A, Schmidt DK, Schultz F, Fricke N, Staden T, Hellweg R et al (2015) Drug and exercise treatment of Alzheimer disease and mild cognitive impairment: a systematic review and meta-analysis of effects on cognition in randomized controlled trials. Am J Geriatr Psychiatry 23(12):1234–1249
Di Santo SG, Prinelli F, Adorni F, Caltagirone C, Musicco M (2013) A meta-analysis of the efficacy of donepezil, rivastigmine, galantamine, and memantine in relation to severity of Alzheimer’s disease. J Alzheimer’s Dis 35(2):349–361
Shilyansky C, Williams LM, Gyurak A, Harris A, Usherwood T, Etkin A (2016) Effect of antidepressant treatment on cognitive impairments associated with depression: a randomised ongitudinal study. Lancet Psychiatry 3(5):425–435. https://doi.org/10.1016/S2215-0366(16)00012-2
Keefe RSE, Buchanan RW, Marder SR, Schooler NR, Dugar A, Zivkov M et al (2013) Clinical trials of potential cognitive-enhancing drugs in schizophrenia: what have we learned so far? Schizophr Bull 39(2):417–435
Cotman CW, Berchtold NC, Christie LA (2007) Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 30(9):464–472
Voss MW, Vivar C, Kramer AF, van Praag H (2013) Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci 17(10):525–544
Schmalzl L, Powers C, Henje Blom E (2015) Neurophysiological and neurocognitive mechanisms underlying the effects of yoga-based practices: towards a comprehensive theoretical framework. Front Hum Neurosci 9:Article 235
Veronese N, Solmi M, Basso C, Smith L, Soysal P (2018) Role of physical activity in ameliorating neuropsychiatric symptoms in Alzheimer disease: a narrative review. Int J Geriatr Psychiatry
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2009) Identifying and quantifying heterogeneity. Introd Meta Anal 2009:107–125 (Proof Review)
Acknowledgements
The authors gratefully acknowledge the corresponding authors of the studies that provided additional information upon request. See Online Resource 3 for a detailed list of author names.
Funding
This study was partly supported by ZONMW TOP Grant 40-00812-98-13009.
Author information
Authors and Affiliations
Contributions
MD, corresponding author, was involved in designing the study, literature search and data collection, performed data analysis, led manuscript preparation and discussion of the results, wrote the manuscript and prepared all the figures and tables and contributed to the manuscript revision. MJHB was involved in data analysis and contributed to the manuscript revision. MIES was involved in literature search and contributed to the manuscript revision. EHML was involved in literature search and contributed to the manuscript revision. PS contributed to the manuscript revision. IES was involved in designing the study, data analysis, discussion of the results, contributed to the manuscript revision and supervised the study. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dauwan, M., Begemann, M.J.H., Slot, M.I.E. et al. Physical exercise improves quality of life, depressive symptoms, and cognition across chronic brain disorders: a transdiagnostic systematic review and meta-analysis of randomized controlled trials. J Neurol 268, 1222–1246 (2021). https://doi.org/10.1007/s00415-019-09493-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09493-9