Abstract
Objective
To evaluate the effect of discontinuation of different disease-modifying therapies (DMTs) before pregnancy with respect to the occurrence of relapses and pregnancy outcomes.
Methods
Women with multiple sclerosis who desire to bear children were followed prospectively. Demographic data, clinical characteristics, and the information on the use of DMTs were collected. A multivariate analysis was used to assess the relationship between relapses and the prior use of different DMTs.
Results
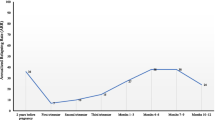
The present study assessed 75 consecutive pregnancy plans (66 women), 65 of which resulted in pregnancy. The mean age of the participants was 32.1 ± 4.2 years, and the mean disease duration was 6.1 ± 4.2 years. No relapses before pregnancy were reported in the group of women who maintained their DMT until pregnancy confirmation, while 14 relapses were reported in 12/42 women (29%) who discontinued DMT before pregnancy. During pregnancy, patients on natalizumab or fingolimod before pregnancy had a higher rate of relapses. Most women restarted their previous DMT after delivery within the first trimester. The relapse rate in postpartum was 0.07.
Conclusions
Disease-modifying therapies received influences the risk of relapse and disease progression from women who are planning pregnancy. The risk of relapse during pregnancy was significantly higher in the group of women treated with natalizumab or fingolimod compared to the group of women treated with interferon beta or glatiramer acetate. The postpartum risk of relapses was lower than that found in previous reports.


Similar content being viewed by others
References
Butler M, Forte ML, Schwehr N, Carpenter A, Kane RL (2015) Decisional Dilemmas in Discontinuing Prolonged Disease-Modifying Treatment for Multiple Sclerosis. Rockville (MD): Agency for Healthcare Research and Quality (US). Report No.: 15-EHC012-EF
Hutchinson M (2011) Safety first, efficacy second in disease modifying therapies. Mult Scler 17:380–381. https://doi.org/10.1177/1352458511402114
Novi G, Ghezzi A, Pizzorno M et al (2017) Dramatic rebounds of MS during pregnancy following fingolimod withdrawal. Neurol Neuroimmunol Neuroinflamm 4(5):e377. https://doi.org/10.1212/NXI.0000000000000377 (eCollection 2017 Sep)
Sempere AP, Berenguer-Ruiz L, Feliu-Rey E (2013) Rebound of disease activity during pregnancy after withdrawal of fingolimod. Eur J Neurol 20:e109–e110. https://doi.org/10.1111/ene.12195
Kleerekooper I, van Kempen ZLE, Leurs CE et al (2018) Disease activity following pregnancy-related discontinuation of natalizumab in MS. Neurol Neuroimmunol Neuroinflamm 5:e424. https://doi.org/10.1212/NXI.0000000000000424
Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T (1998) Rate of pregnancy-related relapse in multiple sclerosis: pregnancy in multiple sclerosis group. N Engl J Med 339:285–291
Vukusic S, Marignier R (2015) Multiple sclerosis and pregnancy in the “treatment era”. Nat Rev Neurol 11:280–289. https://doi.org/10.1038/nrneurol.2015.53
Fragoso YD, Boggild M, Macias-Islas MA et al (2013) The effects of long-term exposure to disease-modifying drugs during pregnancy in multiple sclerosis. Clin Neurol Neurosurg 115:154–159. https://doi.org/10.1016/j.clineuro.2012.04.024
Hellwig K (2019) We need to conduct clinical trials of disease-modifying therapy in pregnancy to optimize care of women with MS. Mult Scler J 25:189–190. https://doi.org/10.1177/1352458518795398
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302. https://doi.org/10.1002/ana.22366
Kalincik T, Cutter G, Spelman T et al (2015) Defining reliable disability outcomes in multiple sclerosis. Brain 138:3287–3298. https://doi.org/10.1093/brain/awv258
WHO (2008) International statistical classification of diseases and related health problems—10th revision, 2008th edn. WHO, Geneva, pp 152–154
Houtchens MK, Edwards NC, Phillips AL (2018) Relapses and disease-modifying drug treatment in pregnancy and live birth in US women with MS. Neurology 2018(91):e1570–e1578. https://doi.org/10.1212/WNL.0000000000006382
Alroughani R, Alowayesh MS, Ahmed SF, Behbehani R, Al-Hashel J (2018) Relapse occurrence in women with multiple sclerosis during pregnancy in the new treatment era. Neurology 90:e840–e846. https://doi.org/10.1212/WNL.0000000000005065
Hatcher SE, Waubant E, Nourbakhsh B, Crabtree-Hartman E, Graves JS (2016) Rebound syndrome in patients with multiple sclerosis after cessation of fingolimod treatment. JAMA Neurol 73:790–794. https://doi.org/10.1001/jamaneurol.2016.0826
Haghikia A, Langer-Gould A, Rellensmann G et al (2014) Natalizumab use during the third trimester of pregnancy. JAMA Neurol 71:891–895. https://doi.org/10.1001/jamaneurol.2014.209
Thiel S, Langer-Gould A, Rockhoff M et al (2016) Interferon-beta exposure during first trimester is safe in women with multiple sclerosis: a prospective cohort study from the German Multiple Sclerosis and Pregnancy Registry. Mult Scler 2016(22):801–809. https://doi.org/10.1177/1352458516634872
Herbstritt S, Langer-Gould A, Rockhoff M et al (2016) Glatiramer acetate during early pregnancy: a prospective cohort study. Mult Scler J 22:810–816. https://doi.org/10.1177/1352458515623366
Breedveld F, Agarwal S, Yin M et al (2007) Rituximab pharmacokinetics in patients with rheumatoid arthritis: B-cell levels do not correlate with clinical response. J Clin Pharmacol 47:1119–1128
Mabthera SmPC (2019). https://www.ema.europa.eu/en/documents/product-information/mabthera-epar-product-information_en.pdf. Accessed 12 Jun 2019.
Das G, Damotte V, Gelfand JM et al (2018) Rituximab before and during pregnancy: a systematic review, and a case series in MS and NMOSD. Neurol. Neuroimmunol Neuroinflamm 2018(5):e453. https://doi.org/10.1212/NXI.0000000000000453
Vukusic S, Hutchinson M, Hours M et al (2004) Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain 127:1353–1360
Hughes SE, Spelman T, Gray OM et al (2014) Predictors and dynamics of postpartum relapses in women with multiple sclerosis. Mult Scler 2014(20):739–746. https://doi.org/10.1177/1352458513507816
Portaccio E, Ghezzi A, Hakiki B et al (2014) Postpartum relapses increase the risk of disability progression in multiple sclerosis: the role of disease modifying drugs. J Neurol Neurosurg Psychiatry 85:846–851. https://doi.org/10.1136/jnnp-2013-306054
Acknowledgements
The authors thank Dr. Santiago Mola and Dr. Arantxa Alfaro for their assistance with data acquisition.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Leticia Berenguer-Ruiz has received personal compensation for consulting, serving on a scientific advisory board or speaking with Almirall, Biogen Idec, Merck Serono, Novartis, Sanofi-Aventis and Teva. Juana Gimenez-Martinez has received speaker honoraria from Almirall, Biogen Idec and Sanofi-Aventis. Antonio Palazón-Bru reports no disclosures. Angel P. Sempere has received personal compensation for consulting, serving on a scientific advisory board or speaking with Almirall, Biogen Idec, Bayer Schering Pharma, Merck Serono, Novartis, Roche, Sanofi-Aventis and Teva.
Ethical standard
The authors confirm that this article complies with ethical standards.
Rights and permissions
About this article
Cite this article
Berenguer-Ruiz, L., Gimenez-Martinez, J., Palazón-Bru, A. et al. Relapses and obstetric outcomes in women with multiple sclerosis planning pregnancy. J Neurol 266, 2512–2517 (2019). https://doi.org/10.1007/s00415-019-09450-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09450-6