Abstract
Background
Cerebrospinal fluid (CSF) biomarkers are increasingly used to diagnose Alzheimer’s disease (AD). However, important methodological and technical remain regarding measurement variability between kit providers and users. We compared the Lumipulse fully automated assays with the manual INNOTEST assays (both from Fujirebio Europe NV, Gent, Belgium) on a clinically representative sample of patients and controls.
Methods
CSF samples of 156 patients were used to quantify Amyloïd Aβ1–42 peptide (Aβ1–42) and Total-Tau (T-Tau) protein by chemiluminescent enzyme-immunoassay (Lumipulse). Patients were divided into several subgroups: Alzheimer (AD = 44), mild-cognitive impairment (MCI = 23), other dementias (OD = 36), non-dementing neurological conditions (ND = 11), and controls (CTRL = 42). Clinical cut-offs were determined by comparing AD and CTRL with ROC curves for the two markers and their related ratio (T-Tau/Aβ1–42). Subgroups of 58 (for phosphorylated-Tau) and 115 samples (for Aβ1–42 and T-Tau) were used to evaluate the concordance of this analyzer with the INNOTEST assays.
Results
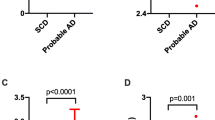
Lumipulse and INNOTEST assays showed good concordance for all markers, but systematic bias was observed justifying the need to redefine new clinical cut-offs. To discriminate AD from CTRL subjects, T-Tau/Aβ1–42 ratio was the best biomarker, with a cut-off value of 1.12 (sensitivity 81.8% and specificity 92.9%). Similar clinical performances were observed for the Lumipulse and Innotests assays on the subsample of 115 subjects.
Conclusions
Our results demonstrate that the Lumipulse Aβ1–42 and T-Tau assays show good analytical and clinical performances in the context of patient evaluation referred to a memory clinic. Automated analyzers should be preferred for the measurement of CSF AD biomarkers to reduce inter- and intra-laboratory variability.



Similar content being viewed by others
References
Wu Y-T, Fratiglioni L, Matthews FE, Lobo A, Breteler MM, Skoog I, Brayne C (2016) Dementia in western Europe: epidemiological evidence and implications for policy making. Lancet Neurol 15(1):116–124
Reitz C, Brayne C, Mayeux R (2011) Epidemiology of Alzheimer disease. Nat Rev Neurol 7(3):137
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297(5580):353–356
Vlassenko AG, McCue L, Jasielec MS, Su Y, Gordon BA, Xiong C, Holtzman DM, Benzinger TL, Morris JC, Fagan AM (2016) Imaging and cerebrospinal fluid biomarkers in early preclinical Alzheimer disease. Ann Neurol 80(3):379–387
Blennow K, Zetterberg H (2018) Biomarkers for Alzheimer's disease: current status and prospects for the future. J Intern Med 284(6):643–663
Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R (2014) Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol 13(6):614–629
Mattsson N, Blennow K, Zetterberg H (2010) Inter-laboratory variation in cerebrospinal fluid biomarkers for Alzheimer's disease: united we stand, divided we fall. Clin Chem Lab Med 48(5):603–607
Verwey N, van der Flier W, Blennow K, Clark C, Sokolow S, De Deyn PP, Galasko D, Hampel H, Hartmann T, Kapaki E (2009) A worldwide multicentre comparison of assays for cerebrospinal fluid biomarkers in Alzheimer's disease. Ann Clin Biochem 46(3):235–240
Fourier A, Portelius E, Zetterberg H, Blennow K, Quadrio I, Perret-Liaudet A (2015) Pre-analytical and analytical factors influencing Alzheimer's disease cerebrospinal fluid biomarker variability. Clin Chim Acta 449:9–15
Del Campo M, Mollenhauer B, Bertolotto A, Engelborghs S, Hampel H, Simonsen AH, Kapaki E, Kruse N, Le Bastard N, Lehmann S (2012) Recommendations to standardize preanalytical confounding factors in Alzheimer’s and Parkinson’s disease cerebrospinal fluid biomarkers: an update. Biomark Med 6(4):419–430
Mattsson N, Lönneborg A, Boccardi M, Blennow K, Hansson O, For the Roadmap GTF (2017) Clinical validity of cerebrospinal fluid Aβ42, tau, and phospho-tau as biomarkers for Alzheimer's disease in the context of a structured 5-phase development framework. Neurobiol Aging 52:196–213
Andreasson U, Kuhlmann J, Pannee J, Umek RM, Stoops E, Vanderstichele H, Matzen A, Vandijck M, Dauwe M, Leinenbach A (2018) Commutability of the certified reference materials for the standardization of β-amyloid 1–42 assay in human cerebrospinal fluid: lessons for tau and β-amyloid 1–40 measurements. Clin Chem Lab Med 56(12):2058–2066
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dement 7(3):263–269
Teunissen CE, Petzold A, Bennett JL, Berven FS, Brundin L, Comabella M, Franciotta D, Frederiksen JL, Fleming JO, Furlan R, Hintzen RQ, Hughes SG, Johnson MH, Krasulova E, Kuhle J, Magnone MC, Rajda C, Rejdak K, Schmidt HK, van Pesch V, Waubant E, Wolf C, Giovannoni G, Hemmer B, Tumani H, Deisenhammer F (2009) A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 73(22):1914–1922. https://doi.org/10.1212/WNL.0b013e3181c47cc2
Vanmechelen E, Vanderstichele H, Davidsson P, Van Kerschaver E, Van Der Perre B, Sjögren M, Andreasen N, Blennow K (2000) Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: a sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci Lett 285(1):49–52
Duits FH, Teunissen CE, Bouwman FH, Visser P-J, Mattsson N, Zetterberg H, Blennow K, Hansson O, Minthon L, Andreasen N (2014) The cerebrospinal fluid “Alzheimer profile”: easily said, but what does it mean? Alzheimer's Dement 10 (6):713–723.e712
Fagan AM, Shaw LM, Xiong C, Vanderstichele H, Mintun MA, Trojanowski JQ, Coart E, Morris JC, Holtzman DM (2011) Comparison of analytical platforms for cerebrospinal fluid measures of β-amyloid 1–42, total tau, and p-tau181 for identifying Alzheimer disease amyloid plaque pathology. Arch Neurol 68(9):1137–1144
Li G, Sokal I, Quinn J, Leverenz J, Brodey M, Schellenberg G, Kaye J, Raskind M, Zhang J, Peskind E (2007) CSF tau/Aβ42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology 69(7):631–639
Fischer P, Jungwirth S, Zehetmayer S, Weissgram S, Hoenigschnabl S, Gelpi E, Krampla W, Tragl K (2007) Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology 68(4):288–291
Davatzikos C, Bhatt P, Shaw LM, Batmanghelich KN, Trojanowski JQ (2011) Prediction of MCI to AD conversion, via MRI, CSF biomarkers, and pattern classification. Neurobiol Aging 32 (12):2322.e2319–2322.e2327
Dumurgier J, Vercruysse O, Paquet C, Bombois S, Chaulet C, Laplanche J-L, Peoc’h K, Schraen S, Pasquier F, Touchon J (2013) Intersite variability of CSF Alzheimer’s disease biomarkers in clinical setting. Alzheimer's Dement 9(4):406–413
Parnetti L, Chiasserini D, Eusebi P, Giannandrea D, Bellomo G, De Carlo C, Padiglioni C, Mastrocola S, Lisetti V, Calabresi P (2012) Performance of Aβ 1–40, Aβ 1–42, total tau, and phosphorylated tau as predictors of dementia in a cohort of patients with mild cognitive impairment. J Alzheimer's Dis 29(1):229–238
Mulder C, Verwey NA, van der Flier WM, Bouwman FH, Kok A, van Elk EJ, Scheltens P, Blankenstein MA (2010) Amyloid-β (1–42), total tau, and phosphorylated tau as cerebrospinal fluid biomarkers for the diagnosis of Alzheimer disease. Clin Chem 56(2):248–253
Dumurgier J, Schraen S, Gabelle A, Vercruysse O, Bombois S, Laplanche J-L, Peoc’h K, Sablonnière B, Kastanenka KV, Delaby C (2015) Cerebrospinal fluid amyloid-β 42/40 ratio in clinical setting of memory centers: a multicentric study. Alzheimer's Res Therapy 7(1):30
De Reuck J, Deramecourt V, Cordonnier C, Pasquier F, Leys D, Maurage C-A, Bordet R (2016) The incidence of post-mortem neurodegenerative and cerebrovascular pathology in mixed dementia. J Neurol Sci 366:164–166
Zhang XY, Yang ZL, Lu GM, Yang GF, Zhang LJ (2017) PET/MR imaging: New frontier in Alzheimer's disease and other dementias. Front Mol Neurosci 10:343
Skillbäck T, Farahmand BY, Rosén C, Mattsson N, Nägga K, Kilander L, Religa D, Wimo A, Winblad B, Schott JM (2015) Cerebrospinal fluid tau and amyloid-β1-42 in patients with dementia. Brain 138(9):2716–2731
Zetterberg H (2017) Tau in biofluids–relation to pathology, imaging and clinical features. Neuropathol Appl Neurobiol 43(3):194–199
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest related to this work.
Ethical standards
The study protocol was in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration. Residual samples used for diagnostic procedures can be used for retrospective academic studies, without any additional informed consent.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bayart, JL., Hanseeuw, B., Ivanoiu, A. et al. Analytical and clinical performances of the automated Lumipulse cerebrospinal fluid Aβ42 and T-Tau assays for Alzheimer’s disease diagnosis. J Neurol 266, 2304–2311 (2019). https://doi.org/10.1007/s00415-019-09418-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09418-6