Abstract
Background
Amyotrophic lateral sclerosis (ALS) is characterized by a spectrum of phenotypes, but only a few studies have addressed the presence of parkinsonian (PK) symptoms. The aim of our study was to investigate the occurrence of PK features in a prospective population-based cohort of ALS patients, determining their demographic, clinical, neuropsychological and genetic characteristics, and identifying their morphological and functional imaging correlates.
Methods
A consecutive series of ALS patients were enrolled and prospectively followed for 2 years. Patients were classified according to the presence (ALS-PK) or absence (ALS) of PK signs, and they underwent neuropsychological testing, genetic analysis for the main ALS and PD genes, brain MRI and 18F-FDG-PET. ALS-PK patients underwent 123I-ioflupane SPECT.
Results
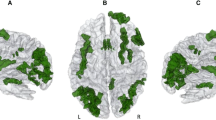
Out of 114 eligible patients, 101 (64 men; mean age at onset 65.1 years) were recruited. Thirty-one patients (30.7%) were classified as ALS-PK. Compared to ALS patients, ALS-PK patients were more frequently male, but did not differ for any other clinical, demographic or neuropsychological factors. 123I-ioflupane SPECT was normal in all but two ALS-PK patients. At 18F-FDG-PET, ALS-PK patients showed a relative hypometabolism in left cerebellum and a relatively more preserved metabolism in right insula and frontal regions; MRI fractional anisotropy was reduced in the sagittal stratum and increased in the retrolenticular part of the internal capsule.
Conclusions
In our study, about 30% of ALS patients showed PK signs. Neuroimaging data indicate that PK signs are due to the involvement of brain circuitries other than classical nigrostriatal ones, strengthening the hypothesis of ALS as a complex multisystem disease.




Similar content being viewed by others
Abbreviations
- ALS:
-
Amyotrophic lateral sclerosis
- PD:
-
Parkinson’s disease
- PARALS:
-
Piemonte and Valle d’Aosta Register for ALS
- FTD:
-
Frontotemporal dementia
- ALS-PK:
-
ALS patients with co-morbid parkinsonian disorder
References
van Es MA, Hardiman O, Chio A, Al-Chalabi A, Pasterkamp RJ, Veldink JH, van den Berg LH (2017) Amyotrophic lateral sclerosis. Lancet 390(10107):2084–2098
Phukan J, Elamin M, Bede P, Jordan N, Gallagher L, Byrne S, Lynch C, Pender N, Hardiman O (2012) The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. J Neurol Neurosurg Psychiatry 83(1):102–108
Montuschi A, Iazzolino B, Calvo A, Moglia C, Lopiano L, Restagno G, Brunetti M, Ossola I, Lo Presti A, Cammarosano S et al (2015) Cognitive correlates in amyotrophic lateral sclerosis: a population-based study in Italy. J Neurol Neurosurg Psychiatry 86(2):168–173
Al-Chalabi A, Hardiman O, Kiernan MC, Chio A, Rix-Brooks B, van den Berg LH (2016) Amyotrophic lateral sclerosis: moving towards a new classification system. Lancet Neurol 15(11):1182–1194
Brait K, Fahn S, Schwarz GA (1973) Sporadic and familial parkinsonism and motor neuron disease. Neurology 23(9):990–1002
Pradat PF, Bruneteau G, Munerati E, Salachas F, Le Forestier N, Lacomblez L, Lenglet T, Meininger V (2009) Extrapyramidal stiffness in patients with amyotrophic lateral sclerosis. Mov Disord 24(14):2143–2148
Pupillo E, Bianchi E, Messina P, Chiveri L, Lunetta C, Corbo M, Filosto M, Lorusso L, Marin B, Mandrioli J et al (2015) Extrapyramidal and cognitive signs in amyotrophic lateral sclerosis: a population based cross-sectional study. Amyotroph Lateral Scler Frontotemporal Degener 16(5–6):324–330
Brooks BR, Miller RG, Swash M, Munsat TL (2000) World federation of neurology research group on motor neuron D: El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1(5):293–299
Chio A, Calvo A, Moglia C, Mazzini L, Mora G (2011) group Ps: phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry 82(7):740–746
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30(12):1591–1601
Ling H, Kara E, Bandopadhyay R, Hardy J, Holton J, Xiromerisiou G, Lees A, Houlden H, Revesz T (2013) TDP-43 pathology in a patient carrying G2019S LRRK2 mutation and a novel p.Q124E MAPT. Neurobiol Aging 34(12):2889 (e2885–2889)
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R et al (2008) Movement disorder society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23(15):2129–2170
Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH, Murphy J, Shoesmith C, Rosenfeld J, Leigh PN et al (2009) Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 10(3):131–146
Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M et al (2013) Criteria for the diagnosis of corticobasal degeneration. Neurology 80(5):496–503
Pinkhardt EH, Sperfeld AD, Gdynia HJ, Ludolph AC, Kassubek J (2009) The combination of dopa-responsive parkinsonian syndrome and motor neuron disease. Neurodegener Dis 6(3):95–101
Williams TL, Shaw PJ, Lowe J, Bates D, Ince PG (1995) Parkinsonism in motor neuron disease: case report and literature review. Acta Neuropathol 89(3):275–283
Desai J, Swash M (1999) Extrapyramidal involvement in amyotrophic lateral sclerosis: backward falls and retropulsion. J Neurol Neurosurg Psychiatry 67(2):214–216
Zoccolella S, Palagano G, Fraddosio A, Russo I, Ferrannini E, Serlenga L, Maggio F, Lamberti S, Iliceto G (2002) ALS-plus: 5 cases of concomitant amyotrophic lateral sclerosis and parkinsonism. Neurol Sci 23(Suppl 2):S123–S124
Manno C, Lipari A, Bono V, Taiello AC, La Bella V (2013) Sporadic Parkinson disease and amyotrophic lateral sclerosis complex (Brait-Fahn-Schwartz disease). J Neurol Sci 326(1–2):104–106
Belin J, Gordon PH, Guennoc AM, De Toffol B, Corcia P (2015) Brait-Fahn-Schwarz disease: the missing link between ALS and Parkinson’s disease. Amyotroph Lateral Scler Frontotemporal Degener 16(1–2):135–136
Gilbert RM, Fahn S, Mitsumoto H, Rowland LP (2010) Parkinsonism and motor neuron diseases: twenty-seven patients with diverse overlap syndromes. Mov Disord 25(12):1868–1875
Boeve BF, Boylan KB, Graff-Radford NR, DeJesus-Hernandez M, Knopman DS, Pedraza O, Vemuri P, Jones D, Lowe V, Murray ME et al (2012) Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain 135(Pt 3):765–783
Theuns J, Verstraeten A, Sleegers K, Wauters E, Gijselinck I, Smolders S, Crosiers D, Corsmit E, Elinck E, Sharma M et al (2014) Global investigation and meta-analysis of the C9orf72 (G4C2)n repeat in Parkinson disease. Neurology 83(21):1906–1913
Borasio GD, Linke R, Schwarz J, Schlamp V, Abel A, Mozley PD, Tatsch K (1998) Dopaminergic deficit in amyotrophic lateral sclerosis assessed with [I-123] IPT single photon emission computed tomography. J Neurol Neurosurg Psychiatry 65(2):263–265
Takahashi H, Snow BJ, Bhatt MH, Peppard R, Eisen A, Calne DB (1993) Evidence for a dopaminergic deficit in sporadic amyotrophic lateral sclerosis on positron emission scanning. Lancet 342(8878):1016–1018
Hideyama T, Momose T, Shimizu J, Tsuji S, Kwak S (2006) A positron emission tomography study on the role of nigral lesions in parkinsonism in patients with amyotrophic lateral sclerosis. Arch Neurol 63(12):1719–1722
Teune LK, Renken RJ, de Jong BM, Willemsen AT, van Osch MJ, Roerdink JB, Dierckx RA, Leenders KL (2014) Parkinson’s disease-related perfusion and glucose metabolic brain patterns identified with PCASL-MRI and FDG-PET imaging. NeuroImage Clinical 5:240–244
Holtbernd F, Ma Y, Peng S, Schwartz F, Timmermann L, Kracht L, Fink GR, Tang CC, Eidelberg D, Eggers C (2015) Dopaminergic correlates of metabolic network activity in Parkinson’s disease. Hum Brain Mapp 36(9):3575–3585
Teune LK, Bartels AL, de Jong BM, Willemsen AT, Eshuis SA, de Vries JJ, van Oostrom JC, Leenders KL (2010) Typical cerebral metabolic patterns in neurodegenerative brain diseases. Mov Disord 25(14):2395–2404
Zheng Z, Shemmassian S, Wijekoon C, Kim W, Bookheimer SY, Pouratian N (2014) DTI correlates of distinct cognitive impairments in Parkinson’s disease. Hum Brain Mapp 35(4):1325–1333
Worker A, Blain C, Jarosz J, Chaudhuri KR, Barker GJ, Williams SC, Brown RG, Leigh PN, Dell’Acqua F, Simmons A (2014) Diffusion tensor imaging of Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy: a tract-based spatial statistics study. PLoS One 9(11):e112638
Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM (2007) White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 130(Pt 10):2508–2519
Acknowledgements
The Project has been supported by Italian Ministry of Health (Ricerca Finalizzata Giovani Ricercatori 2010; GR-2010-2320550, PI AndC); AndC thanks ‘Vialli e Mauro Foundation’; AntC thanks ‘Magnetto Foundation’. This study was supported by Ministero dell’Istruzione, dell’Università e della Ricerca—MIUR project “Dipartimenti di Eccellenza 2018–2022” to ‘Rita Levi Montalcini’ Department of Neuroscience.
Author information
Authors and Affiliations
Contributions
AC, AC, LL and MGR contributed to the fund raising, literature search, figures, study design, data collection, data analysis, data interpretation, writing, and revision of the manuscript. AC, AC, SC, FD, UM, CM, AR, BI and EM contributed to the data collection, data analysis, and revision of the manuscript. LS, UM, CAA and AC contributed to writing, data analysis and revision of the manuscript. MP, QT and FN contributed to data analysis, data interpretation, and revision of the manuscript. TM, MB, GC, MB, AC, GC and CV contributed to data collection, data analysis, data interpretation, and revision of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Andrea Calvo reports no disclosure. Adriano Chiò reports personal fees from Biogen Idec, Cytokinetics, Mitsubishi Tanabe, and Neuraltus, outside the submitted work. Marco Pagani reports no disclosure. Stefania Cammarosano reports no disclosure. Francesca Dematteis reports no disclosure. Cristina Moglia reports no disclosure. Luca Solero reports no disclosure. Umberto Manera reports no disclosure. Tiziana Martone reports no disclosure. Maura Brunetti reports no disclosure. Michele Balma reports no disclosure. Giancarlo Castellano reports no disclosure. Marco Barberis reports no disclosure. Angelina Cistaro reports no disclosure. Carlo Alberto Artusi reports no disclosure. Elisa Montanaro reports no disclosure. Alberto Romagnolo reports no disclosure. Barbara Iazzolino reports no disclosure. Antonio Canosa reports no disclosure. Giovanna Carrara reports no disclosure. Consuelo Valentini reports no disclosure. Tie-Qiang Li reports no disclosure. Flavio Nobili reports no disclosure. Leonardo Lopiano reports no disclosure. Mario G. Rizzone reports no disclosure.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Calvo, A., Chiò, A., Pagani, M. et al. Parkinsonian traits in amyotrophic lateral sclerosis (ALS): a prospective population-based study. J Neurol 266, 1633–1642 (2019). https://doi.org/10.1007/s00415-019-09305-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09305-0