Abstract
Diagnostic accuracy is crucial not only for prognostic and therapeutic reasons, but also for epidemiologic studies. We aimed to study the accuracy of the clinical diagnosis of Parkinson disease (PD) for participants in The Nord-Trøndelag Health Study (HUNT), a health survey, containing data from approximately 126,000 individuals and biological material from 80,000 individuals. We included 980 participants from the HUNT study diagnosed with PD or secondary parkinsonism/related parkinsonian disorders. The participants had been diagnosed in conjunction with admission to hospitals in Trøndelag or through out-patient examination. We validated the diagnosis of PD by reviewing available Electronic Health Records (EHRs) using the MDS Clinical Diagnostic Criteria as gold standard. In total 61% (601/980) of the participants had available EHRs and were selected for validation. Out of those, 92% (550/601) had been diagnosed with PD while 8% (51/601) had been diagnosed with secondary parkinsonism/related parkinsonian disorders. The main outcome measure was the accuracy of the clinical diagnosis of PD for participants in the HUNT study. We verified PD in 65% (358/550) and excluded PD in 35% (192/550) of the participants. According to our results, the overall quality of the clinical diagnosis of PD for participants in the HUNT study is not optimal. Quality assurance of ICD codes entered into health registers is crucial before biological material obtained from these populations can be used in the search of new biomarkers for PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson disease (PD) is a progressive neurological disorder characterized by large numbers of motor and non-motor features which causes a heavy burden both on those affected, as well as their families. We mainly base the diagnosis of PD on clinical criteria. Definite diagnosis, however, are obtained only pathologically [1]. Correct diagnosis is crucial for prognostic and therapeutic reasons, but also for epidemiologic studies [2]. Population health data sets are helpful tools to evaluate health outcomes and health services as well as to describe the clinical course of disease and the total burden of disease and interventions in the population. They can provide data for the assessment of risk factors, causal pathways, positive and negative correlations with other diseases and rare outcomes in addition to provide accurate measures of disease, which, in turn, can define incidence and prevalence of a disease [3]. However, diagnosis-coding error is a challenge. According to O’Malley et al. [4], there are main sources of diagnostic coding error both along the ‘‘patient trajectory’’ (the dynamic interplay between the patient as he or she progresses through the health care system) and the ‘‘paper trail’’ (the creation of the medical record). Main sources of error along the ‘‘patient trajectory’’ include “the amount and quality of information at admission, communication among patients and providers, the clinician’s knowledge and experience with the illness and the clinician’s attention to detail. Main sources of error along the ‘‘paper trail’’ include “variance in the electronic and written records, coder training and experience, facility quality-control efforts and not intentional and intentional coder errors.
The Nord-Trøndelag Health Study (HUNT) was conducted in three waves of data gathering and biological sampling, the HUNT1 Survey (1984–1986), the HUNT2 Survey (1995–1997) and the HUNT3 Survey (2006–2008). Today, the HUNT study databank contains health survey data from approximately 126,000 individuals, both family data and individual data, which can be linked to national health registries. Biological material was collected from all participants (80,000) in the HUNT2 and HUNT3 surveys. The diagnosis of PD can be straightforward when patients have a classical presentation. However, differentiating PD from related parkinsonian disorders early in the course of the disease, when signs and symptoms overlap with other syndromes, may be challenging [5]. The aims of our study were to assess the accuracy of the clinical diagnosis of PD for participants in the HUNT study.
Method
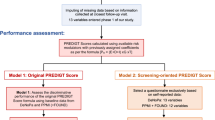
We identified all participants in the HUNT study who had been diagnosed with parkinsonism by comparing the eleven-digit unique personal identification number (PIN) of the participants who had taken part in at least one HUNT Survey to the official registry of PD diagnosis during the same period. The diagnoses were set at hospitals in Trøndelag. The following ICD codes were used: (ICD 10)/332 (ICD 9) (PD), G21 (secondary parkinsonism), G21.1 (drug-induced secondary parkinsonism), G21.2 (secondary parkinsonism due to other external causes), G 21.3 (post encephalitic parkinsonism), G21.8 (another secondary parkinsonism), G21.9 (unspecified secondary parkinsonism), G23 (other degenerative diseases of basal ganglia), G23.1 (progressive supra nuclear palsy), G23.9 (unspecified degenerative disease of the basal ganglia) and G25.8 (other special extrapyramidal conditions).
Cardinal signs of PD are asymmetric bradykinesia, rest tremor and rigidity. To differentiate PD from related parkinsonian disorders, we use the presence and specific presentation of the cardinal signs. Additional clinical features include secondary motor symptoms (hypomimia, dysarthria, dysphagia, sialorrhoea, micrographia, shuffling gait, festination, postural instability, freezing, dystonia, glabellar reflexes) and non-motor symptoms (autonomic dysfunction, cognitive/neurobehavioral abnormalities, sleep disorders and sensory abnormalities such as anosmia, paresthesias and pain) [6]. “Absence of rest tremor, early occurrence of gait difficulty, postural instability, dementia, hallucinations, and the presence of dysautonomia, ophthalmoparesis, ataxia and other atypical features, coupled with poor or no response to levodopa, suggest diagnoses other than PD” [6].
Since there is no definitive test for the diagnosis of PD, we based the diagnosis on the MDS Clinical Diagnostic Criteria for PD [7]. We reviewed Electronic Health Records (EHRs) of the participants in the HUNT study and classified them according to these criteria. If not seen by an experienced neurologist, the PD diagnosis were accepted only if the EHRs contained sufficient information on tremor, bradykinesia and rigidity. The long-term follow-up effect of levodopa was also emphasized.
We classified participants as probable PD if the EHRs described parkinsonism (defined as bradykinesia in combination with at least one of rest tremor or rigidity), combined with at least two supportive criteria (excellent response to levodopa, presence of levodopa-induced dyskinesia or rest tremor of a limb documented on clinical examination) without no absolute exclusion criteria or red flags. If the EHRs described parkinsonism combined with presence of up to two red flags counterbalanced by supportive criteria and absence of absolute exclusion criteria, we classified participants as possible PD. Participants without EHRs were excluded since this would require review of Paper Health Records in at least two, sometimes three hospitals in the region, which we considered too laborious. Main outcome measure was the accuracy of the clinical diagnosis of PD for participants in the HUNT study.
This study was approved by the Ethics Committee of Central Norway, and informed consent was obtained from all participants.
Results
In total, we identified 980 participants in the HUNT study who had been diagnosed with parkinsonism. However, we excluded 39% (379/980) due to lack of EHRs. Participants in the HUNT1 Survey only, accounted for the majority of those (75%, 263/349) as shown in Table 1. Of the participants with EHRs, 92% (550/601) had been diagnosed with PD G20 (ICD 10) /332 (ICD 9) while 8% (51/601) had been diagnosed with secondary parkinsonism G 21.1, G 21.2, G 21.3, G 21.8, G 21.9 or related parkinsonian disorders G 23.1, G 23. 9. We verified PD (probable PD and possible PD) in 65% (358/550) and excluded PD (not PD) in 35% (192/550), as shown in Table 2. Among the participants we classified as not PD, we found a related parkinsonian disorder and secondary parkinsonism distribution as shown in Table 3. Figure 1 illustrating the distribution of age at disease onset, shows predominantly older age at disease onset in the “possible PD” group compared to the “probable PD” group. The majority of participants, 71% (428/601), had been diagnosed by a neurologist, and only a few 8% (49/601) by a movement disorder expert. About 21% (124/601) had been diagnosed during hospitalization in medical, surgical or psychiatric ward. In 8% we lack information about where the diagnosis was set.
Discussion
The aims of our study were to assess the accuracy of the clinical diagnosis of PD for participants in the HUNT study. This was a diagnosis validation study, as opposed to an audit of coding practices. The diagnostic accuracy in our study, 65%, is consistent with the results previously reported by Rizzo et al. [2] who evaluated diagnostic accuracy of clinical diagnosis of PD, reported in the last 25 years by a systematic review and meta-analysis. According to their results, the accuracy was 73.8% for clinical diagnosis performed mainly by non-experts and 79.6% for clinical diagnosis performed by movement disorder experts, rising from 79.6% of initial assessment to 83.9% of refined diagnosis after follow-up. Mean disease duration at last evaluation was 10.2 years, with range from 3.6 to 13.8 years [2].
There are numerous possibilities for incorrect diagnostic coding both in the “patient trajectory” and the ‘‘paper trail’’ [4]. One important source of error is the misdiagnosis made by the physicians. Low diagnostic accuracy is particularly relevant in the earliest stages of parkinsonism and presumably in older patients, even for movement disorder experts. This is due to the absence of hallmarks of atypical parkinsonism and less defined response to dopaminergic treatment [2]. Figure 1, illustrating the distribution of age at disease onset in HUNT participants, shows predominantly older age at disease onset in the “possible PD” group compared to the “probable PD” group.
The clinical diagnosis of PD was revised in a number of patients at follow-up due to lack of observed efficacy of levodopa or development of additional atypical features. However, the misdiagnosis was still present in the EHRs, which may cause incorrect selection of patients when performing diagnosis specific search in the registries. Rizzo et al. [2] reports a disease duration ranging from 3.6 to 13.8 years (mean 10.2 years) as useful to increase the diagnostic accuracy, without a clear trend of improvement of the accuracy over time. In trials, incorrect encoding may cause recruitment of participants without the trial specific diagnosis and exclusion of candidates. This may be a challenge, particularly in clinical trials designed to recruit participants with early PD [2].
Both unintentional and intentional coder errors, such as misspecification, unbundling, and up-coding, are additional potential sources of errors [4]. Since we have limited knowledge about the diagnostic coding process in hospitals in Trøndelag, we cannot estimate the level of intentional or unintentional coder errors that may have occurred. However, based on a clear discrepancy between the diagnosis registered in the contact section of the EHRs and the one posed and recorded by the physician, cf. medical journal text, we found 9% (21/240) coder errors [8].
We consider lack of access to medical records for all participants as well as diagnostic validation based on EHRs of widely varying quality a limitation of our study [9, 10]. However, we have no indication that participants in the HUNT1 Survey only, were diagnosed with higher accuracy than those participating in the later HUNT surveys. Among participants with verified PD, the rate of participation in the HUNT surveys decreased as the disease progressed causing participation (response) bias and/or self-selection bias [11].
The HUNT study has several strengths. It is covering a total population aged 20–100 years within a geographical area, which includes inland and coastal municipalities with varying characteristics. Since the age range is wide, groups of people with different cohort exposures are covered. The databank includes data on an extensive range of topics (> 6000 variables) where all data are linked to the unique PIN, which enables linkage of data for each of the participants and linkage to numbers of local, regional and national health registers, with nearly complete follow-up data [12]. The HUNT study has several advantages for epidemiologic and genetic research including cost efficiency, the large amounts of available clinical data, and the ability to analyze data over time [13]. Electronic phenotypes extracted from EHRs and other registries can be paired with genetic data to identify genes underlying common human diseases [14]. However, diagnostic accuracy is crucial when combining clinical data from health information databases to the genetic data.
This study demonstrates that the overall quality of the clinical diagnosis of PD for participants in the HUNT study is not optimal. The ICD codes entered into health registers must be quality-assured before biological material obtained from these populations can be used in the search for new biomarkers for PD. Incorrect diagnosis of cases and controls may give biased effect size estimates and misleading association results.
References
Braak H et al (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211
Rizzo G et al (2016) Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology 86(6):566–576
Roberts CL et al (2008) The accuracy of reporting of the hypertensive disorders of pregnancy in population health data. Hypertens Pregnancy 27(3):285–297
O’Malley KJ et al (2005) Measuring diagnoses: ICD code accuracy. Health Serv Res 40(5 Pt 2):1620–1639
Tolosa E, Wenning G, Poewe W (2006) The diagnosis of Parkinson’s disease. Lancet Neurol 5(1):75–86
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79(4):368–376
Postuma RB et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30(12):1591–1601
Mikkelsen G, Aasly J (2003) Narrative electronic patient records as source of discharge diagnoses. Comput Methods Programs Biomed 71(3):261–268
Mikkelsen G, Aasly J (2005) Consequences of impaired data quality on information retrieval in electronic patient records. Int J Med Inform 74(5):387–394
Mikkelsen G, Aasly J (2001) Concordance of information in parallel electronic and paper based patient records. Int J Med Inform 63(3):123–131
Lacey JV Jr, Savage KE (2016) 50% Response rates: half-empty, or half-full? Cancer Causes Control 27(6):805–808
Krokstad S et al (2013) Cohort profile: the HUNT study, Norway. Int J Epidemiol 42(4):968–977
Wei WQ, Denny JC (2015) Extracting research-quality phenotypes from electronic health records to support precision medicine. Genome Med 7(1):41
Beaulieu-Jones BK, Greene CS (2016) Semi-supervised learning of the electronic health record for phenotype stratification. J Biomed Inform 64:168–178
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interests.
Ethical approval
The study was approved by the Ethics Committee of Central Norway.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hustad, E., Skogholt, A.H., Hveem, K. et al. The accuracy of the clinical diagnosis of Parkinson disease. The HUNT study. J Neurol 265, 2120–2124 (2018). https://doi.org/10.1007/s00415-018-8969-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8969-6