Abstract
Introduction
The mechanism of action of fingolimod within the central nervous system and its efficacy in reducing/preventing both focal and diffuse grey matter (GM) damage in active multiple sclerosis (MS) are not completely understood.
Methods
In this longitudinal, 2-year prospective, phase IV, single-blind study, 40 MS patients treated with fingolimod and 39 untreated age, gender, and disability-matched MS patients were enrolled. Each patient underwent a neurological examination every 6 months and a 3T MRI at the beginning of the treatment and after 24 months. The accumulation of new cortical lesions (CLs) and the progression of regional GM atrophy were compared between the two groups.
Results
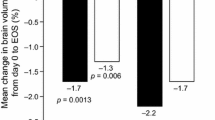
At the end of the study (T24), the percentage of patients with new CLs (13.5 vs. 89%, p < 0.001) and the percentage of GM volume change was lower in the treated group (p < 0.001). The regional analysis revealed that the treated group had also less volume loss in thalamus, caudatus, globus pallidus, cingulate cortex, and hippocampus (p < 0.001), as well as in, cerebellum, superior frontal gyrus, and insular-long gyrus (p < 0.05). Patients with no evidence of disease activity were 60% in the treated group and 10% in the untreated group (p < 0.001).
Conclusions
These results suggest a possible protective effect of fingolimod on focal and diffuse GM damage.



Similar content being viewed by others
References
Noseworthy JH, Lucchinetti C, Rodriguez M et al (2000) Multiple sclerosis. N Engl J Med 343:938–952. https://doi.org/10.1056/NEJM200009283431307
Trapp BD, Peterson J, Ransohoff RM et al (1998) Axonal transection in the lesions of multiple sclerosis. N Engl J Med 338:278–285. https://doi.org/10.1056/NEJM199801293380502
Peterson JW, Bö L, Mörk S et al (2001) Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 50:389–400
Calabrese M, Romualdi C, Poretto V et al (2013) The changing clinical course of multiple sclerosis: a matter of gray matter. Ann Neurol 74:76–83. https://doi.org/10.1002/ana.23882
Calabrese M, Poretto V, Favaretto A et al (2012) Cortical lesion load associates with progression of disability in multiple sclerosis. Brain 135:2952–2961. https://doi.org/10.1093/brain/aws246
Calabrese M, Magliozzi R, Ciccarelli O et al (2015) Exploring the origins of grey matter damage in multiple sclerosis. Nat Rev Neurosci 16:147–158. https://doi.org/10.1038/nrn3900
Kappos L, Radue E-W, O’Connor P et al (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362:387–401. https://doi.org/10.1056/NEJMoa0909494
Cohen JA, Barkhof F, Comi G et al (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362:402–415. https://doi.org/10.1056/NEJMoa0907839
Oo ML, Thangada S, Wu M-T et al (2007) Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem 282:9082–9089. https://doi.org/10.1074/jbc.M610318200
Pelletier D, Hafler DA (2012) Fingolimod for multiple sclerosis. N Engl J Med 366:339–347. https://doi.org/10.1056/NEJMct1101691
Pitteri M, Magliozzi R, Bajrami A et al (2018) Potential neuroprotective effect of Fingolimod in multiple sclerosis and its association with clinical variables. Expert Opin Pharmacother 19:387–395. https://doi.org/10.1080/14656566.2018.1434143
Foster CA, Howard LM, Schweitzer A et al (2007) Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental autoimmune encephalomyelitis: consequences for mode of action in multiple sclerosis. J Pharmacol Exp Ther 323:469–475. https://doi.org/10.1124/jpet.107.127183
Mullershausen F, Zecri F, Cetin C et al (2009) Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol 5:428–434. https://doi.org/10.1038/nchembio.173
Colombo E, Di Dario M, Capitolo E et al (2014) Fingolimod may support neuroprotection via blockade of astrocyte nitric oxide. Ann Neurol 76:325–337. https://doi.org/10.1002/ana.24217
Hoffmann FS, Hofereiter J, Rübsamen H et al (2015) Fingolimod induces neuroprotective factors in human astrocytes. J Neuroinflamm 12:184. https://doi.org/10.1186/s12974-015-0393-6
Cui QL, Fang J, Kennedy TE et al (2014) Role of p38MAPK in S1P receptor-mediated differentiation of human oligodendrocyte progenitors. Glia 62:1361–1375. https://doi.org/10.1002/glia.22688
Cipriani R, Chara JC, Rodríguez-Antigüedad A et al (2017) Effects of FTY720 on brain neurogenic niches in vitro and after kainic acid-induced injury. J Neuroinflamm 14:147. https://doi.org/10.1186/s12974-017-0922-6
Landi D, Vollaro S, Pellegrino G et al (2015) Oral fingolimod reduces glutamate-mediated intracortical excitability in relapsing–remitting multiple sclerosis. Clin Neurophysiol 126:165–169. https://doi.org/10.1016/j.clinph.2014.05.031
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33(11):1444–1452
Geurts JJG, Roosendaal SD, Calabrese M et al (2011) Consensus recommendations for MS cortical lesion scoring using double inversion recovery MRI. Neurology 76:418–424. https://doi.org/10.1212/WNL.0b013e31820a0cc4
Geurts JJG, Bö L, Pouwels PJW et al (2005) Cortical lesions in multiple sclerosis: combined postmortem MR imaging and histopathology. AJNR Am J Neuroradiol 26:572–577
Seewann A, Vrenken H, Kooi E-J et al (2011) Imaging the tip of the iceberg: visualization of cortical lesions in multiple sclerosis. Mult Scler J 17:1202–1210. https://doi.org/10.1177/1352458511406575
Seewann A, Kooi E-J, Roosendaal SD et al (2012) Postmortem verification of MS cortical lesion detection with 3D DIR. Neurology 78:302–308. https://doi.org/10.1212/WNL.0b013e31824528a0
Sethi V, Yousry T, Muhlert N et al (2016) A longitudinal study of cortical grey matter lesion subtypes in relapse-onset multiple sclerosis. J Neurol Neurosurg Psychiatry 87:750–753. https://doi.org/10.1136/jnnp-2015-311102
Havrdova E, Galetta S, Stefoski D et al (2010) Freedom from disease activity in multiple sclerosis. Neurology 74(Suppl 3):S3–S7. https://doi.org/10.1212/WNL.0b013e3181dbb51c
Giovannoni G, Turner B, Gnanapavan S, Offiah C, Schmierer K, Marta M (2015) Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord 4(4):329–333. https://doi.org/10.1016/j.msard.2015.04.006
Gaetano L, Häring DA, Radue E-W et al (2018) Fingolimod effect on gray matter, thalamus, and white matter in patients with multiple sclerosis. Neurology 90:e1324–e1332. https://doi.org/10.1212/WNL.0000000000005292
Calabrese M, Bernardi V, Atzori M et al (2012) Effect of disease-modifying drugs on cortical lesions and atrophy in relapsing–remitting multiple sclerosis. Mult Scler J 18:418–424. https://doi.org/10.1177/1352458510394702
Barkhof F, de Jong R, Sfikas N et al (2014) The influence of patient demographics, disease characteristics and treatment on brain volume loss in trial assessing injectable interferon vs FTY720 oral in relapsing–remitting multiple sclerosis (TRANSFORMS), a phase 3 study of fingolimod in multiple sclerosis. Mult Scler 20:1704–1713. https://doi.org/10.1177/1352458514532317
Calabresi P, Radue E-W, Goodin D et al (2014) Safety and efficacy of fingolimod in patients with relapsing–remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 13:545–556. https://doi.org/10.1016/S1474-4422(14)70049-3
Claes N, Dhaeze T, Fraussen J et al (2014) Compositional changes of B and T CELL subtypes during fingolimod treatment in multiple sclerosis patients: a 12-month follow-up study. PLoS One 9:e111115. https://doi.org/10.1371/journal.pone.0111115
Brinkmann V, Billich A, Baumruker T et al (2010) Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov 9:883–897. https://doi.org/10.1038/nrd3248
Lucchinetti CF, Popescu BFG, Bunyan RF et al (2011) Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 365:2188–2197. https://doi.org/10.1056/NEJMoa1100648
De Stefano N, Matthews PM, Filippi M et al (2003) Evidence of early cortical atrophy in MS: relevance to white matter changes and disability. Neurology 60:1157–1162
Ceccarelli A, Rocca MA, Pagani E et al (2008) A voxel-based morphometry study of grey matter loss in MS patients with different clinical phenotypes. Neuroimage 42:315–322. https://doi.org/10.1016/j.neuroimage.2008.04.173
Audoin B, Zaaraoui W, Reuter F et al (2010) Atrophy mainly affects the limbic system and the deep grey matter at the first stage of multiple sclerosis. J Neurol Neurosurg Psychiatry 81:690–695. https://doi.org/10.1136/jnnp.2009.188748
Geurts JJG, Bö L, Roosendaal SD et al (2007) Extensive hippocampal demyelination in multiple sclerosis. J Neuropathol Exp Neurol 66:819–827. https://doi.org/10.1097/nen.0b013e3181461f54
Calabrese M, Castellaro M, Bertoldo A et al (2017) Epilepsy in multiple sclerosis: the role of temporal lobe damage. Mult Scler J 23:473–482. https://doi.org/10.1177/1352458516651502
Giovannoni G, Tomic D, Bright JR, Havrdová E (2017) “No evident disease activity”: the use of combined assessments in the management of patients with multiple sclerosis. Mult Scler 23(9):1179–1187. https://doi.org/10.1177/1352458517703193
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The local Ethic Committee approved the present retrospective study and the informed consent was obtained from all patients.
Conflicts of interest
I certify that all authors have seen, reviewed, and agreed with the content of the manuscript and that the submission is not under review at any other publication. No submissions and previous reports are overlapping with the current work. Massimiliano Calabrese received payment for development of educational presentations including service on speakers’ bureaus by Biogen-Elan, Genzyme, TEVA, and Bayer-Schering, he received travel/accommodation expenses by Novartis Pharma, Genzyme, Biogen idec, Merck Serono, Bayer-Schering, and TEVA, and Advisory Board membership by Bayer-Shering, Genzyme, Biogen Idec and Novartis Pharma. All the other authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bajrami, A., Pitteri, M., Castellaro, M. et al. The effect of fingolimod on focal and diffuse grey matter damage in active MS patients. J Neurol 265, 2154–2161 (2018). https://doi.org/10.1007/s00415-018-8952-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8952-2