Abstract
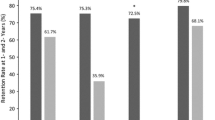
The first objective was to determine the long-term retention rate of eight antiepileptic drugs (AEDs) commonly used as adjunctive therapy in adults with focal refractory epilepsy. Second, we assessed the effects of age and gender on retention rates. Third, we examined if the retention rate could be influenced by the sequence in which the AEDs had entered the market. Patients with focal refractory epilepsy treated with any of the eight AEDs in Tampere University Hospital were identified retrospectively (N = 507). Retention rates were evaluated with the Kaplan–Meier method. Follow-up started at the first date of treatment and each individual was followed a maximum of 36 months. We calculated the following 3-year retention rates: lacosamide 77.1% (N = 137), lamotrigine 68.3% (N = 177), levetiracetam 66.7% (N = 319), clobazam 65.6% (N = 130), topiramate 61.6% (N = 178), zonisamide 60.4% (N = 103), pregabalin 54.6% (N = 127), and gabapentin 40.2% (N = 66). Lacosamide, levetiracetam, and clobazam were the most effective AEDs in the elderly. The retention rate for pregabalin was higher in males (65%) than females (51%) whereas females had higher retention rates for both topiramate (72 vs. 58%) and zonisamide (67 vs. 57%). The retention rate was influenced by the sequence in which these AEDs entered the market. We provide important information about practical aspects of these eight AEDs, revealing that there are differences in their effectiveness as adjunctive treatment for focal refractory epilepsy. Most importantly, the retention rate appears to be influenced by the sequence in which these AEDs were introduced onto the market.


Similar content being viewed by others
References
French JA, Faught E (2009) Rational polytherapy. Epilepsia 50(Suppl. 8):63–68
Sander JW (2005) New antiepileptic drugs in clinical practice—how do they perform in the real world? Acta Neurol Scand Suppl 181:26–29
ILAE Commission on Antiepileptic Drugs (1998) Report of the ILAE commission on antiepileptic drugs: considerations on designing clinical trials to evaluate the place of new antiepileptic drugs in the treatment of newly diagnosed and chronic patients with epilepsy. Epilepsia 39:799–803
European Medicines Agency (EMEA), Committee for Medicinal Products for Human Use (CHMP) (2010) Guideline on clinical investigation of medicinal products in the treatment of epileptic disorders. CHMP/EWP/566/98/Rev. 2/Corr
Ben-Menachem E, Sander JW, Privitera M, Gilliam F (2010) Measuring outcomes of treatment with antiepileptic drugs in clinical trials. Epilepsy Behav 18:24–30
Bootsma HP, Ricker L, Hekster YA, Hulsman J, Lambrechts D, Majoie M, Schellekens A, De Krom M, Aldenkamp AP (2009) The impact of side effects on long-term retention in three new antiepileptic drugs. Seizure 18:327–331
Rainesalo S, Peltola J, Auvinen A, Keränen T (2005) Retention rate of oxcarbazepine monotherapy in an unselected population of adult epilepsies. Seizure 14:72–74
Peltola J, Peltola M, Auvinen A, Raitanen J, Fallah M, Keränen T (2009) Retention rates of new antiepileptic drugs in localization-related epilepsy: a single center study. Acta Neur Scand 119:55–60
Arif H, Buchsbaum R, Pierro J, Whalen M, Sims J, Resor SR Jr, Bazil CW, Hirchs LJ (2009) Comparative effectiveness of 10 antiepileptic drugs in older adults with epilepsy. Arch Neurol 67:408–415
Zaccara G, Messori A, Cincotta M, Burchini G (2006) Comparison of the efficacy and tolerability of new antiepileptic drugs: what can we learn from long-term studies? Acta Neur Scand 114:157–168
Scheffer IE, French J, Hirsch E, Jain S, Mathern GW, Moshé SL, Perucca E, Tomson T, Wiebe S, Zhang Y-H, Zuberi SM (2016) Classification of epilepsies: new concepts for discussion and debate—Special report of the ILAE, classification task force of the commission for classification and terminology. Epilepsia Open 1:37–44
Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Hauser AW, Mathern G, Moshé SL, Perucca E, Wiebe S, French J (2010) Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 51:1069–1077
Ben-Menachem E (2007) Weight issues for people with epilepsy—a review. Epilepsia Suppl 48:42–45
Stephen LJ, Kelly K, Parker P, Brodie MJ (2014) Adjunctive lacosamide—5 years’ clinical experience. Epilepsy Res 108:1385–1391
Novy J, Bartolini E, Bell GS, Duncan JS, Sander JW (2013) Long-term retention rate of lacosamide in a large cohort of people with medically refractory epilepsy: a single center evaluation. Epilepsy Res 106:250–256
Kamel JT, DeGruyter MA, D‘Souza WJ, Cook MJ (2013) Clinical experience with using lacosamide for the treatment of epilepsy in a tertiary centre. Acta Neurol Scand 127:149–153
Villanueva V, López-Gomáriz E, López-Trigo J, Palau J, García M, Villarroya T, Bonet M, Santafé C (2012) Rational polytherapy with lacosamide in clinical practice: results of a Spanish cohort analysis RELACOVA. Epilepsy Behav 23:298–304
Chung S, Wang N, Hank N (2007) Comparative retention rates and long-term tolerability of new antiepileptic drugs. Seizure 16:296–304
Lhatoo SD, Wong IC, Polizzi G, Sander JW (2000) Long-term retention rates of lamotrigine, gabapentin, and topiramate in chronic epilepsy. Epilepsia 41:1592–1596
Brodie MJ, Kelly K, Stephen LJ (2014) Prospective audits with newer antiepileptic drugs in focal epilepsy: insights into population responses? Epilepsy Behav 31:73–76
Montenegro MA, Arif H, Nahm EA, Resor SR Jr, Hirch LJ (2008) Efficacy of clobazam as add-on therapy for refractory epilepsy: experience at a US epilepsy center. Clin Neuropharmacol 31:333–338
Collins TL, Petroff OA, Mattson RH (2000) A comparison of four new antiepileptic medications. Seizure 9:291–293
Bootsma HPR, Ricker L, Diepman L, Gehring J, Hulsman J, Lambrechts D, Leenen L, Majoie M, Schellekens A, de Krom M, Aldenkamp AP (2008) Long-term effects of levetiracetam and topiramate in clinical practice: a head-to-head comparison. Seizure 17:19–26
Nakken KO, Lindstrøm P, Andersen H (2015) Retention rate of zonisamide in intractable epilepsy. Acta Neur Scand 131:268–274
Catarino CB, Bartolino E, Bell GS, Yuen AW, Duncan JS, Sander JW (2011) The long-term retention of zonisamide in a large cohort of people with epilepsy at a tertiary referral center. Epilepsy Res 96:39–44
Yuen AW, Singh R, Bell GS, Bhattacharjee A, Neligan A, Heaney DC, Duncan JS, Sander JW (2009) The long-term retention of pregabalin in a large cohort of patients with epilepsy at tertiary referral center. Epilepsy Res 87:120–123
Stephen LJ, Kelly K, Wilson EA, Parker P, Brodie MJ (2010) A prospective audit of adjunctive zonisamide in an everyday clinical setting. Epilepsy Behav 17:455–460
Stephen LJ, Parker P, Kelly K, Wilson EA, Leach V, Brodie MJ (2011) Adjunctive pregabalin for uncontrolled partial-onset seizures: findings from a prospective audit. Acta Neurol Scand 124:142–145
Liimatainen SP, Raitanen JA, Ylinen AM, Peltola MA, Peltola JT (2008) The benefit of active drug trials is dependent on aetiology in refractory focal epilepsy. J Neurol Neurosurg Psychiatry 79:808–812
WHO Collaborating Centre for Drug Statistics Methodology (2016) ATC/DDD index 2016. http://www.whocc.no/atc_ddd_index/. Accessed 26 May 2017
Acknowledgements
All authors meet the International Committee of Medical Journals Editors (ICMJE) criteria for authorship and have given final approval to the manuscript to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
This was a non-invasive, retrospective study, which does not oblige ethics committee approval according to Finnish Law on Research. Access to patient records based on decision made by Head of Science Centre, Tampere University Hospital research and innovation services, Science Center.
Conflicts of interest and sources of funding statement
Jussi Mäkinen has received support for travel congresses from Biogen-Idec, Boehringer-Ingelheim, Eisai, and Orion Pharma; received speaker honoraria from Boehringer-Ingelheim; received research funding from the Finnish Epilepsy Association and Maire Taponen Foundation; and participated in an advisory board for Eisai. Jani Raitanen has no conflict of interest. Sirpa Rainesalo has received speaker honoraria from FennoMedical, Orion Pharma, UCB and received support for travel to congresses from Abbvie and UCB. Tiina Alapirtti has received support for travel to congresses from Biogen, AbbVie, Biogen, Genzyme, Roche, and UCB. Jukka Peltola has participated in clinical trials for Eisai, UCB, and Bial; received research grants from Eisai, Medtronic, UCB, and Cyberonics; received speaker honoraria from Cyberonics, Eisai, Medtronic, Orion Pharma, and UCB; received support for travel congresses from Cyberonics, Eisai, Medtronic, and UCB; and participated in advisory boards for Cyberonics, Eisai, Medtronic, UCB, and Pfizer.
Rights and permissions
About this article
Cite this article
Mäkinen, J., Peltola, J., Raitanen, J. et al. Comparative effectiveness of eight antiepileptic drugs in adults with focal refractory epilepsy: the influence of age, gender, and the sequence in which drugs were introduced onto the market. J Neurol 264, 1345–1353 (2017). https://doi.org/10.1007/s00415-017-8526-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8526-8