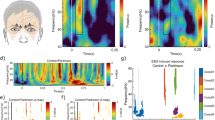
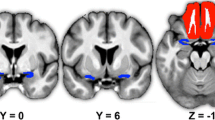
Abstract
Early diagnosis and timely treatment of Parkinson’s disease are essential factors to provide these patients with a longer period of a better quality of life. Olfactory loss is among the first non-motor symptoms of the disease; however, in light of the many causes of smell loss, it is a very unspecific biomarker and should only be used as part of a diagnostic test battery. In this study, we investigated the olfactory response in 71 subjects, consisting of Parkinson’s disease patients, hyposmic and anosmic patients of other causes, and normosmic individuals searching for sensitive, distinct biomarkers for which we used scalp event-related 64-channel electroencephalography and psychophysical tests. The analysis of the global field power indicated significant measurable differences between patients with Parkinson’s disease and otherwise olfactory dysfunctional and normosmic individuals. The localization of brain sources, in particular, provides evidence for differences in mainly late EEG-components suggesting a decline of central brain networks as a causal factor for olfactory loss in Parkinson’s disease. The findings indicate a different pattern of olfactory processing in patients with Parkinson’s disease compared to olfactory dysfunctions of other origin, which provide further insights into the mechanisms behind olfactory dysfunction in Parkinson’s.




Similar content being viewed by others
References
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F (1973) Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20(4):415–455
Berg D, Postuma RB, Adler CH et al (2015) MDS research criteria for prodromal Parkinson’s disease. Mov Disord 30(12):1600–1611
Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318(1):121–134
Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H (2002) Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol 61(5):413–426
Haehner A, Boesveldt S, Berendse HW et al (2009) Prevalence of smell loss in Parkinson’s disease—a multicenter study. Parkinsonism Relat Disord 15(7):490–494
Doty RL, Shaman P, Dann M (1984) Development of the University of Pennsylvania smell identification test: a standardized microencapsulated test of olfactory function. Physiol Behav 32(3):489–502
Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G (1997) ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22(1):39–52
Mueller A, Reichman H, Livermore A, Hummel T (2002) Olfactory function in idiopathic Parkinson’s disease: results from cross-sectional studies in IPD patients and long-term follow-up of de-novo IPD patients. J Neural Transm 109:805–811
Wenning GK, Shephard B, Hawkes C, Petruckevitch A, Lees A, Quinn N (1995) Olfactory function in atypical parkinsonian syndromes. Acta Neurol Scand 91(4):247–250
Whitcroft KL, Cuevas M, Haehner A, Hummel T (2017) Patterns of olfactory impairment reflect underlying disease etiology. Laryngoscope 127(2):291–295
Hummel T, Kobal G (2001) Olfactory event-related potentials. In: Simon SA, Nicolelis MAL (eds) Methods and frontiers in chemosensory research. CRC Press, Boca Raton, pp 129–164
Harada H, Shiraishi K, Kato T (2003) Olfactory event-related potentials in normal subjects and patients with smell disorders. Clin Electroencephalogr 34(4):191–196
Picton TW, Lins OG, Scherg M (1994) Handbook of neurophysiology. Elsevier, Amsterdam
Picton TW, Hillyard SA, Krausz HI, Galambos R (1974) Human auditory evoked potentials. I. Evaluation of components. Electroencephalogr Clin Neurophysiol 36(2):179–190
Kobal G, Plattig KH (1978) Objective olfactometry: methodological annotations for recording olfactory EEG-responses from the awake human. EEG EMG Z Elektroenzephalogr Elektromyogr Verwandte Geb 9(3):135–145
Iannilli E, Beger M, Furer R, Hummel T (2015) A gustatory stimulator. J Neurosci Methods 255:12–16
Barz S, Hummel T, Pauli E, Majer M, Lang CJ, Kobal G (1997) Chemosensory event-related potentials in response to trigeminal and olfactory stimulation in idiopathic Parkinson’s disease. Neurology 49(5):1424–1431
Hawkes CH, Shephard BC, Daniel SE (1997) Olfactory dysfunction in Parkinson’s disease. J Neurol Neurosurg Psychiatry 62(5):436–446
Bocquillon P, Bourriez JL, Palmero-Soler E, Defebvre L, Derambure P, Dujardin K (2015) Impaired early attentional processes in Parkinson’s disease: a high-resolution event-related potentials study. PLoS One 10(7):e0131654
Solis-Vivanco R, Rodriguez-Violante M, Rodriguez-Agudelo Y, Schilmann A, Rodriguez-Ortiz U, Ricardo-Garcell J (2015) The P3a wave: a reliable neurophysiological measure of Parkinson’s disease duration and severity. Clin Neurophysiol 126(11):2142–2149
Martinez-Horta S, Riba J, de Bobadilla RF et al (2014) Apathy in Parkinson’s disease: neurophysiological evidence of impaired incentive processing. J Neurosci 34(17):5918–5926
Mensen A, Poryazova R, Schwartz S, Khatami R (2014) Humor as a reward mechanism: event-related potentials in the healthy and diseased brain. PLoS One 9(1):e85978
Dietz J, Bradley MM, Jones J, Okun MS, Perlstein WM, Bowers D (2013) The late positive potential, emotion and apathy in Parkinson’s disease. Neuropsychologia 51(5):960–966
Desmedt JE, Tomberg C, Noël P, Ozaki I (1990) Beware of the average reference in brain mapping. Electroencephalogr Clin Neurophysiol Suppl 41:22–27
Lehmann D, Skrandies W (1980) Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalogr Clin Neurophysiol 48(6):609–621
Pascual-Marqui RD, Lehmann D (1993) Comparison of topographic maps and the reference electrode: comments on two papers by Desmedt and collaborators. Electroencephalogr Clin Neurophysiol 88(530–531):534–536
Litvan I, Bhatia KP, Burn DJ et al (2003) Movement Disorders Society Scientific Issues Committee report: SIC task force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord 18(5):467–486
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198
Kobal G, Klimek L, Wolfensberger M et al (2000) Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. Eur Arch Otorhinolaryngol 257(4):205–211
Peirce JW (2007) PsychoPy–psychophysics software in Python. J Neurosci Methods 162(1–2):8–13
Halliday AM, Halliday E, Kriss A, McDonald WI, Mushin J (1976) The pattern-evoked potential in compression of the anterior visual pathways. Brain 99(2):357–374
Bodis-Wollner I, Yahr MD (1978) Measurements of visual evoked potentials in Parkinson’s disease. Brain 101(4):661–671
Bodis-Wollner I, Yahr MD, Mylin L, Thornton J (1982) Dopaminergic deficiency and delayed visual evoked potentials in humans. Ann Neurol 11(5):478–483
Peppe A, Stanzione P, Pierelli F, De Angelis D, Pierantozzi M, Bernardi G (1995) Visual alterations in de novo Parkinson’s disease: pattern electroretinogram latencies are more delayed and more reversible by levodopa than are visual evoked potentials. Neurology 45(6):1144–1148
Grave de Peralta Menendez R, Gonzalez Andino S, Lantz G, Michel CM, Landis T (2001) Noninvasive localization of electromagnetic epileptic activity. I. Method descriptions and simulations. Brain Topogr 14(2):131–137
Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R (2004) EEG source imaging. Clin Neurophysiol 115(10):2195–2222
Pause BM, Sojka B, Krauel K, Ferstl R (1996) The nature of the late positive complex within the olfactory event-related potential (OERP). Psychophysiology 33(4):376–384
Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P (2001) The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann N Y Acad Sci 935:107–117
Rosenberg-Katz K, Maidan I, Jacob Y, Giladi N, Mirelman A, Hausdorff JM (2016) Alterations in conflict monitoring are related to functional connectivity in Parkinson’s disease. Cortex 82:277–286
Gaspar P, Berger B, Febvret A, Vigny A, Henry JP (1989) Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. J Comp Neurol 279(2):249–271
Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD (1998) Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280(5364):747–749
Kuenig G, Leenders KL, Martin C, Magyar S, Schultz W (1999) Reward processing in Parkinsons disease A PET activation study. Neurology 52(2 suppl 2):A349
Torack RM, Morris JC (1988) The association of ventral tegmental area histopathology with adult dementia. Arch Neurol 45(5):497–501
German DC, Manaye K, Smith WK, Woodward DJ, Saper CB (1989) Midbrain dopaminergic cell loss in Parkinson’s disease: computer visualization. Ann Neurol 26(4):507–514
Rinne JO, Rummukainen J, Paljarvi L, Rinne UK (1989) Dementia in Parkinson’s disease is related to neuronal loss in the medial substantia nigra. Ann Neurol 26(1):47–50
Grossman M, Crino P, Reivich M, Stern MB, Hurtig HI (1992) Attention and sentence processing deficits in Parkinson’s disease: the role of anterior cingulate cortex. Cereb Cortex 2(6):513–525
Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AH (1989) Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol 33(3):161–253
Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL (1984) Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science 225(4667):1168–1170
Summerfield C, Junque C, Tolosa E et al (2005) Structural brain changes in Parkinson disease with dementia: a voxel-based morphometry study. Arch Neurol 62(2):281–285
Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA (2003) Parkinson’s disease is associated with hippocampal atrophy. Mov Disord 18(7):784–790
Xu Y, Yang J, Hu X, Shang H (2016) Voxel-based meta-analysis of gray matter volume reductions associated with cognitive impairment in Parkinson’s disease. J Neurol 263(6):1178–1187
Jahanshahi M, Jones CR, Zijlmans J et al (2010) Dopaminergic modulation of striato-frontal connectivity during motor timing in Parkinson’s disease. Brain 133(Pt 3):727–745
Farrer C, Frey SH, Van Horn JD et al (2008) The angular gyrus computes action awareness representations. Cereb Cortex 18(2):254–261
Seghier ML (2013) The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist 19(1):43–61
Seibyl JP, Marek KL, Quinlan D et al (1995) Decreased single-photon emission computed tomographic [123I]beta-CIT striatal uptake correlates with symptom severity in Parkinson’s disease. Ann Neurol 38(4):589–598
Gruzelier JH (1999) Functional neuropsychophysiological asymmetry in schizophrenia: a review and reorientation. Schizophr Bull 25(1):91–120
Innis RB, Seibyl JP, Scanley BE et al (1993) Single photon emission computed tomographic imaging demonstrates loss of striatal dopamine transporters in Parkinson disease. Proc Natl Acad Sci USA 90(24):11965–11969
Marek KL, Seibyl JP, Zoghbi SS et al (1996) [123I] beta-CIT/SPECT imaging demonstrates bilateral loss of dopamine transporters in hemi-Parkinson’s disease. Neurology 46(1):231–237
Acknowledgements
We would like to express our gratitude to all volunteers, patients, and their families that contributed, with their dedicated participation, to the completion of the demanding tasks in this work. This research did not receive any specific Grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All the listed authors declare no conflict of interest in the conduct and reporting of this research.
Ethical standard
The study has been approved by the Ethics Board of the Medical Faculty of the “Technische Universität Dresden with all aspects of the study performed in accordance with the Declaration of Helsinki. All participants gave written consent. The author hereby declares that the research documented in the submitted manuscript has been carried out in accordance with the above stated ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Iannilli, E., Stephan, L., Hummel, T. et al. Olfactory impairment in Parkinson’s disease is a consequence of central nervous system decline. J Neurol 264, 1236–1246 (2017). https://doi.org/10.1007/s00415-017-8521-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8521-0