Abstract
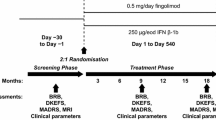
Natalizumab discontinuation is associated with a disease reactivation in multiple sclerosis (MS) patients. Whether this reactivation involves also cognitive functions is not known to date. To assess the persistence of the effect of natalizumab on cognitive functions 1 year after its discontinuation, we compared the longitudinal changes of cognitive performances in two groups of patients. The interrupters, 30 MS patients, have stopped natalizumab due to PML concern, and the continuers, 28 MS patients, continued the treatment. The cognitive impairment index (CII) was used as main outcome measure. As expected, during the natalizumab treatment, we observed a significant reduction of the relapse rate and the number of gadolinium-enhancing lesions along with a reduction of the CII. After 1 year of discontinuation, the beneficial effect on cognitive functions was lost in the interrupters group, as the mean CII increased in comparison with the mean at the end of natalizumab treatment (12.2 ± 7.9 vs 9.3 ± 8.1, p < 0.0001). As opposite, in the continuers group, the CII further decreased after an additional year of treatment (8.4 ± 5.1 vs 9.8 ± 4.6, p = 0.007). A multivariate logistic regression model revealed as predictors of cognitive worsening male sex, disease duration, and the treatment discontinuation. The worsening of cognitive functions after natalizumab discontinuation goes in parallel with the clinical/radiological disease reactivation. Our data reinforce the hypothesis that, in the short-term, natalizumab exerts its positive impact on cognitive functions by means of its anti-inflammatory properties.
Similar content being viewed by others
References
Chiaravalloti ND, DeLuca J (2008) Cognitive impairment in multiple sclerosis. Lancet Neurol 7:1139–1151
Rao SM (1995) Neuropsychology of multiple sclerosis. Curr Opin Neurol 8:216–220
Amato MP, Zipoli V, Portaccio E (2006) Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J Neurol Sci 245:41–46
He D, Zhang Y, Dong S et al (2013) Pharmacological treatment for memory disorder in multiple sclerosis. Cochrane Database Syst Rev 17(12):CD008876
Pliskin NH, Hamer DP, Goldstein DS, Towle VL, Reder AT et al (1996) Improved delayed visual reproduction test performance in multiple sclerosis patients receiving interferon b-1b. Neurology 47:1463–1468
Fischer JS, Priore RL, Jacobs LD, Cookfair DL, Rudick RA et al (2000) Neuropsychological effects of interferon b-1a in relapsing multiple sclerosis. Ann Neurol 48:885–892
Cohen JA, Cutter GR, Fischer JS, Goodman AD, Heidenreich FR et al (2002) Benefit of interferon b-1a on MSFC progression in secondary progressive MS. Neurology 59:679–687
Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP et al (2009) Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol 8:987–997
Patti F, Amato MP, Bastianello S, Caniatti L, Di Monte E et al (2010) Effects of immunomodulatory treatment with subcutaneous interferon beta-1a on cognitive decline in mildly disable patients with relapsing remitting multiple sclerosis. Mult Scler 16:68–77
Mattioli F, Stampatori C, Capra R (2011) The effect of Natalizumab on cognitive function in patients with relapsing-remitting multiple sclerosis: preliminary results of a 1-year follow-up study. Neurol Sci 32:83–88
Iaffaldano P, Viterbo RG, Paolicelli D et al (2012) Impact of Natalizumab on cognitive performances and fatigue in relapsing multiple sclerosis: a prospective, open-label. Two years observational study. PLoS One 7(4):e35843. doi:10.1371/journal.pone.0035843
Kunkel A, Fischer M, Faiss J et al (2015) Impact of Natalizumab treatment on fatigue, mood, and aspects of cognition in relapsing-remitting multiple sclerosis. Front Neurol 11(6):97
Mattioli F, Stampatori C, Bellomi F, Scarpazza C, Capra R (2015) Natalizumab significantly improves cognitive impairment over 3 years in MS: pattern of disability progression and preliminary MRI findings. PLoS One 10(7):e0131803
Killestein J, Vennegoor A, Strijbis EM et al (2010) Natalizumab drug holiday in multiple sclerosis: poorly tolerated. Ann Neurol 68:392–395
West TW, Cree BA (2010) Natalizumab dosage suspension: are we helping or hurting? Ann Neurol 68:395–399
Borriello G, Prosperini L, Marinelli F, Fubelli F, Pozzilli C (2011) Observations during an elective interruption of Natalizumab treatment: a post-marketing study. Mult Scler 17:372–375
Borriello G, Prosperini L, Mancinelli C, Gianni C, Fubelli F, Pozzilli C (2012) Pulse monthly steroids during an elective interruption of Natalizumab: a post-marketing study. Eur J Neurol 19:783–787
Havla J, Gerdes LA, Meinl I et al (2011) De-escalation from Natalizumab in multiple sclerosis: recurrence of disease activity despite switching to glatiramer acetate. J Neurol 258:1665–1669
Kaufman MD, Lee R, Norton HJ (2011) Course of relapsing remitting multiple sclerosis before, during and after Natalizumab. Mult Scler 17:490–494
Kerbrat A, Le Page E, Leray E et al (2011) Natalizumab and drug holiday in clinical practice: an observational study in very active relapsing remitting multiple sclerosis patients. J Neurol Sci 308:98–102
Magraner MJ, Coret F, Navarre A et al (2011) Pulsed steroids followed by glatiramer acetate to prevent inflammatory activity after cessation of Natalizumab therapy: a prospective, 6-month observational study. J Neurol 258:1805–1811
O’Connor PW, Goodman A, Kappos L, Lublin FD, Miller DH, Polman C et al (2011) Disease activity return during Natalizumab treatment interruption in patients with multiple sclerosis. Neurology 76(22):1858–1865
Rossi S, Motta C, Studer V et al (2013) Effect of glatiramer acetate on disease reactivation in MS patients discontinuing Natalizumab. Eur J Neurol 20:87–94
Cohen M, Maillart E, Tourbah A, De Sèze J, Vukusic S, Brassat D et al (2014) Switching from Natalizumab to fingolimod in multiple sclerosis: a French prospective study. JAMA Neurol. doi:10.1001/jamaneurol.2013.6240
Jokubaitis VG, Li V, Kalincik T, Izquierdo G, Hodgkinson S, Alroughani R et al (2014) Fingolimod after Natalizumab and the risk of short-term relapse. Neurology 82(14):1204–1211. doi:10.1212/WNL.0000000000000283
Iaffaldano P, Lucisano G, Pozzilli C, et al. (2015) Fingolimod versus interferon beta/glatiramer acetate after Natalizumab suspension in multiple sclerosis. Brain 138(11):3275–3286. doi:10.1093/brain/awv260
Clerico M, Schiavetti I, De Mercanti SF, Piazza F, Gned D, Brescia Morra V et al (2014) Treatment of relapsing-remitting multiple sclerosis after 24 doses of Natalizumab: evidence from an Italian spontaneous, prospective, and observational study (the TY-STOP Study). JAMA Neurol. doi:10.1001/jamaneurol.2014.1200
Fox RJ, Cree BA, De Sèze J, Gold R, Hartung HP, Jeffery D et al (2014) MS disease activity in RESTORE: a randomized 24-week Natalizumab treatment interruption study. Neurology 82(17):1491–1498
Morrow SA, Jurgensen S, Forrestal F, Munchauer FE, Benedict RH (2011) Effects of acute relapses on neuropsychological status in multiple sclerosis patients. J Neurol 258(9):1603–1608
McGuigan C, Craner M, Guadagno J, Kapoor R, Mazibrada G, Molyneux P et al (2016) Stratification and monitoring of Natalizumab-associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group. J Neurol Neurosurg Psychiatry 87(2):117–125. doi:10.1136/jnnp-2015-311100 (Epub 2015 Oct 22)
Amato MP, Portaccio E, Goretti B, Zipoli V, Ricchiuti L et al (2006) The Rao’s brief repeatable battery and stroop test: normative values with age, education and gender corrections in an Italian population. Mult Scler 12:787–793
Camp SJ, Stevenson VL, Thompson AJ, Miller DH, Borras C et al (1999) Cognitive function in primary progressive and transitional progressive multiple sclerosis. A controlled study with MRI correlates. Brain 122:1341–1348
Amato MP, Goretti B, Ghezzi A, Hakiki B, Niccolai C, Lori S et al (2014) Neuropsychological features in childhood and juvenile multiple sclerosis: five-year follow-up. Neurology 83(16):1432–1438. doi:10.1212/WNL.0000000000000885
Goretti B, Patti F, Cilia S, Mattioli F, Stampatori C et al (2014) The Rao’s Brief Repeatable Battery version B: normative values with age, education and gender corrections in an Italian population. Neurol Sci 35(1):79–82. doi:10.1007/s10072-013-1558-7 Epub 2013 Oct 8
Mandolesi G, Grasselli G, Musumesi G, Centonze D (2010) Cognitive deficits in experimental autoimmune encephalomyelitis: neuroinflammation and synaptic degeration. Neurol Sci 31(Suppl 2):S255–S259
Centonze D, Muzio L, Rossi S, Cavasinni F, De Chiara V et al (2009) Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J Neurosci 29:3442–3452
Gunnarsson M, Malmestro¨m C, Axelsson M, Sundstro¨m P, Dahle C et al (2010) Axonal damage in relapsing multiple sclerosis is markedly reduced by Natalizumab. Ann Neurol 69:83–89
Khademi M, Bornsen L, Rafatnia F, Andersson M, Brundin L et al (2009) The effects of Natalizumab on inflammatory mediators in multiple sclerosis: prospects for treatment-sensitive biomarkers. Eur J Neurol 16:528–536
Mellergard J, Edstrom M, Vrethem M, Erneurdh J, Dahle C (2010) Natalizumab treatment in multiple sclerosis: marked decline of chemokines and cytokines in cerebrospinal fluid. Mult Scler 16:208–217
Iaffaldano P, Ruggieri M, Viterbo RG, Mastrapasqua M, Trojano M (2014) The improvement of cognitive functions is associated with a decrease of plasma osteopontin levels in Natalizumab treated relapsing multiple sclerosis. Brain Behav Immun 35:176–181
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Iaffaldano P. has served on scientific advisory boards for Biogen Idec and Bayer, and has received funding for travel and/or speaker honoraria from Sanofi-Aventis, Biogen Idec, Teva and Novartis. Viterbo R.G. has served on scientific advisory boards for Biogen Idec and has received speaker honoraria from Teva and Novartis. Trojano M. has received honoraria for consultancy or speaking from Biogen, Sanofi-Aventis, Merck Serono and Bayer-Schering and research grants from Merck Serono, Biogen and Novartis.
Rights and permissions
About this article
Cite this article
Iaffaldano, P., Viterbo, R. & Trojano, M. Natalizumab discontinuation is associated with a rebound of cognitive impairment in multiple sclerosis patients. J Neurol 263, 1620–1625 (2016). https://doi.org/10.1007/s00415-016-8177-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8177-1