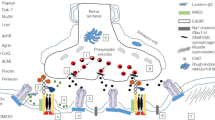
Abstract
Congenital myasthenic syndromes (CMSs) are a group of heterogeneous inherited disorders caused by mutations in genes affecting the function and structure of the neuromuscular junction. This review updates the reader on established and novel subtypes of congenital myasthenia, and the treatment strategies for these increasingly heterogeneous disorders. The discovery of mutations associated with the N-glycosylation pathway and in the family of serine peptidases has shown that causative genes encoding ubiquitously expressed molecules can produce defects at the human neuromuscular junction. By contrast, mutations in lipoprotein-like receptor 4 (LRP4), a long-time candidate gene for congenital myasthenia, and a novel phenotype of myasthenia with distal weakness and atrophy due to mutations in AGRN have now been described. In addition, a pathogenic splicing mutation in a nonfunctional exon of CHRNA1 has been reported emphasizing the importance of analysing nonfunctional exons in genetic analysis. The benefit of salbutamol and ephedrine alone or combined with pyridostigmine or 3,4-DAP is increasingly being reported for particular subtypes of CMS.



Similar content being viewed by others
References
Palace J, Beeson D (2008) The congenital myasthenic syndromes. J Neuroimmunol 201–202:2–5
Belaya K, Finlayson S, Slater CR et al (2012) Mutations in DPAGT1 cause a limb-girdle congenital myasthenic syndrome with tubular aggregates. Am J Hum Genet 91:193–201
Senderek J, Müller JS, Dusl M et al (2011) Hexosamine biosynthetic pathway mutations cause neuromuscular transmission defect. Am J Hum Genet 88:162–172
Cossins J, Belaya K, Hicks D et al (2013) Congenital myasthenic syndromes due to mutations in ALG2 and ALG14. Brain 136:944–956
Chaouch A, Beeson D, Hantaï D, Lochmüller H (2012) 186th ENMC international workshop: congenital myasthenic syndromes 24–26 June 2011, Naarden, The Netherlands. Neuromuscul Disord 22:566–576
Engel AG (2012) Current status of the congenital myasthenic syndromes. Neuromuscul Disord 22:99–111
Finlayson S, Beeson D, Palace J (2013) Congenital myasthenic syndromes: an update. Pract Neurol 13:80–91
Webster R, Maxwell S, Spearman H et al (2012) A novel congenital myasthenic syndrome due to decreased acetylcholine receptor ion-channel conductance. Brain 135:1070–1080
Zhu H, Pytel P, Gomez CM (2014) Selective inhibition of caspases in skeletal muscle reverses the apoptotic synaptic degeneration in slow-channel myasthenic syndrome. Hum Mol Genet 23:69–77
Chaouch A, Müller JS, Guergueltcheva V et al (2012) A retrospective clinical study of the treatment of slow-channel congenital myasthenic syndrome. J Neurol 259:474–481
Webster RG, Cossins J, Lashley D et al (2013) A mouse model of the slow channel myasthenic syndrome: Neuromuscular physiology and effects of ephedrine treatment. Exp Neurol 248:286–298
Palace J, Lashley D, Bailey S et al (2012) Clinical features in a series of fast channel congenital myasthenia syndrome. Neuromuscul Disord 22:112–117
Shen X-M, Brengman JM, Edvardson S et al (2012) Highly fatal fast-channel syndrome caused by AChR ε subunit mutation at the agonist binding site. Neurology 79:449–454
Shen X, Brengman JM, Sine SM, Engel AG (2012) Myasthenic syndrome AChR α C-loop mutant disrupts initiation of channel gating. J Clin Invest 122:2613–2621
Webster R, Liu W-W, Chaouch A et al (2013) Fast-channel congenital myasthenic syndrome with a novel acetylcholine receptor mutation at the α-ε subunit interface. Neuromuscul Disord 24:143–147
Mukhtasimova N, Sine SM (2007) An intersubunit trigger of channel gating in the muscle nicotinic receptor. J Neurosci 27:4110–4119
Rahman MA, Masuda A, Ohe K et al (2013) HnRNP L and hnRNP LL antagonistically modulate PTB-mediated splicing suppression of CHRNA1 pre-mRNA. Sci Rep 3:2931
Park J, Mott M, Williams T, et al. (2014) Neurobiology of disease a single mutation in the acetylcholine receptor δ-subunit causes distinct effects in two types of neuromuscular synapses. 34:10211–10218
Punga AR, Maj M, Lin S et al (2011) MuSK levels differ between adult skeletal muscles and influence postsynaptic plasticity. Eur J Neurosci 33:890–898
DeChiara TM, Bowen DC, Valenzuela DM et al (1996) The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 85:501–512
Mihaylova V, Salih MaM, Mukhtar MM et al (2009) Refinement of the clinical phenotype in musk-related congenital myasthenic syndromes. Neurology 73:1926–1928
Chevessier F, Faraut B, Ravel-Chapuis A et al (2005) Pathophysiological characterization of congenital myasthenic syndromes: the example of mutations in the MUSK gene. J Soc Biol 199:61–77
Maselli Ra, Arredondo J, Cagney O et al (2010) Mutations in MUSK causing congenital myasthenic syndrome impair MuSK-Dok-7 interaction. Hum Mol Genet 19:2370–2379
Gallenmüller C, Felber WM, Dusl M et al (2014) Salbutamol-responsive limb-girdle congenital myasthenic syndrome due to a novel missense mutation and heteroallelic deletion in MUSK. Neuromuscul Disord 24:31–35
Okada K, Inoue A, Okada M et al (2006) The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science 312:1802–1805
Beeson D, Higuchi O, Palace J et al (2006) Dok-7 mutations underlie a neuromuscular junction synaptopathy. Science 313:1975–1978
Klein A, Pitt MC, McHugh JC et al (2013) DOK7 congenital myasthenic syndrome in childhood: early diagnostic clues in 23 children. Neuromuscul Disord 23:883–891
Cossins J, Liu WW, Belaya K et al (2012) The spectrum of mutations that underlie the neuromuscular junction synaptopathy in DOK7 congenital myasthenic syndrome. Hum Mol Genet 21:3765–3775
Witting N, Vissing J (2014) Pharmacologic treatment of downstream of tyrosine kinase 7 congenital myasthenic syndrome. JAMA Neurol 71:350–354
Ohno K, Brengman J, Tsujino a, Engel aG (1998) Human endplate acetylcholinesterase deficiency caused by mutations in the collagen-like tail subunit (ColQ) of the asymmetric enzyme. Proc Natl Acad Sci USA 95:9654–9659
Ito M, Suzuki Y, Okada T et al (2012) Protein-anchoring strategy for delivering acetylcholinesterase to the neuromuscular junction. Mol Ther 20:1384–1392
Nakata T, Ito M, Azuma Y et al (2013) Mutations in the C-terminal domain of ColQ in endplate acetylcholinesterase deficiency compromise ColQ-MuSK interaction. Hum Mutat 34:997–1004
Arredondo J, Lara M, Ng F et al (2013) COOH-terminal collagen Q (COLQ) mutants causing human deficiency of endplate acetylcholinesterase impair the interaction of ColQ with proteins of the basal lamina. Hum Genet 133:599–616
Matlik HN, Milhem RM, Saadeldin IY et al (2014) Clinical and molecular analysis of a novel COLQ missense mutation causing congenital myasthenic syndrome in a Syrian family. Pediatr Neurol 51:165–169
Ohno K, Tsujino a, Brengman JM et al (2001) Choline acetyltransferase mutations cause myasthenic syndrome associated with episodic apnea in humans. Proc Natl Acad Sci USA 98:2017–2022
Schara U, Christen H-J, Durmus H et al (2010) Long-term follow-up in patients with congenital myasthenic syndrome due to CHAT mutations. Eur J Paediatr Neurol 14:326–333
Shen X-M, Crawford TO, Brengman J et al (2011) Functional consequences and structural interpretation of mutations of human choline acetyltransferase. Hum Mutat 32:1259–1267
Kim N, Stiegler AL, Cameron TO et al (2008) Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell 135:334–342
Zhang W, Coldefy A-S, Hubbard SR, Burden SJ (2011) Agrin binds to the N-terminal region of Lrp4 protein and stimulates association between Lrp4 and the first immunoglobulin-like domain in muscle-specific kinase (MuSK). J Biol Chem 286:40624–40630
Ohkawara B, Cabrera-Serrano M, Nakata T et al (2013) LRP4 third β-propeller domain mutations cause novel congenital myasthenia by compromising agrin-mediated musk signaling in a position-specific manner. Hum Mol Genet 23:1856–1868
Richard P, Goillot E, Huze C et al (2009) Identification of an agrin mutation that causes congenital myasthenia and affects synapse function. Am J Hum Genet 85:155–167
Maselli RA, Fernandez JM, Arredondo J et al (2012) LG2 agrin mutation causing severe congenital myasthenic syndrome mimics functional characteristics of non-neural (z-) agrin. Hum Genet 131:1123–1135
Nicole S, Chaouch A, Torbergsen T et al (2014) Agrin mutations lead to a congenital myasthenic syndrome with distal muscle weakness and atrophy. Brain 137:2429–2443
Lone AM, Nolte WM, Tinoco AD, Saghatelian A (2010) Peptidomics of the prolyl peptidases. AAPS J 12:483–491
Martens K, Derua R, Meulemans S et al (2006) PREPL: a putative novel oligopeptidase propelled into the limelight. Biol Chem 387:879–883
Régal L, Shen X-M, Selcen D et al (2014) PREPL deficiency with or without cystinuria causes a novel myasthenic syndrome. Neurology 82:1254–1260
Kim M-H, Hersh LB (2004) The vesicular acetylcholine transporter interacts with clathrin-associated adaptor complexes AP-1 and AP-2. J Biol Chem 279:12580–12587
Radhakrishnan K, Baltes J, Creemers JWM, Schu P (2013) Trans-Golgi network morphology and sorting is regulated by prolyl-oligopeptidase-like protein PREPL and the AP-1 complex subunit μ1A. J Cell Sci 126:1155–1163
Jaeken J, Matthijs G (2001) Congenital disorders of glycosylation. Annu Rev Genomics Hum Genet 2:129–151
Wu X, Rush JS, Karaoglu D et al (2003) Deficiency of UDP-GlcNAc:Dolichol Phosphate N-Acetylglucosamine-1 Phosphate Transferase (DPAGT1) causes a novel congenital disorder of Glycosylation Type I. J Hum Mutat 22:144–150
Thiel C, Schwarz M, Peng J et al (2003) A new type of congenital disorders of glycosylation (CDG-Ii) provides new insights into the early steps of dolichol-linked oligosaccharide biosynthesis. J Biol Chem 278:22498–22505
Haltiwanger RS, Lowe JB (2004) Role of glycosylation in development. Annu Rev Biochem 73:491–537
Velina Guergueltcheva, Jacqueline Palace, David Beeson HL (2011) Congenital myasthenic syndrome with tubular aggregates caused by GFPT1 mutations. J Neurol
Selcen D, Shen X-M, Milone M et al (2013) GFPT1-myasthenia: clinical, structural, and electrophysiologic heterogeneity. Neurology 81:370–378
Huh S-Y, Kim H-S, Jang H-J et al (2012) Limb-girdle myasthenia with tubular aggregates associated with novel GFPT1 mutations. Muscle Nerve 46:600–604
Martin PT (2003) Glycobiology of the neuromuscular junction. J Neurocytol 32:915–929
Zoltowska K, Webster R, Finlayson S et al (2013) Mutations in GFPT1 that underlie limb-girdle congenital myasthenic syndrome result in reduced cell-surface expression of muscle AChR. Hum Mol Genet 22:2905–2913
Bretthauer RK (2009) Structure, expression, and regulation of UDP-GlcNAc: dolichol phosphate GlcNAc-1-phosphate transferase (DPAGT1). Curr Drug Targets 10:477–482
Merlie JP, Sebbane R, Tzartos S, Lindstrom J (1982) Inhibition of glycosylation with tunicamycin blocks assembly of newly synthesized acetylcholine receptor subunits in muscle cells. J Biol Chem 257:2694–2701
Selcen D, Shen X, Brengman J (2014) expression studies DPAGT1 myasthenia and myopathy. Neurology 82:1822–1830
Basiri K, Belaya K, Liu WW et al (2013) Clinical features in a large Iranian family with a limb-girdle congenital myasthenic syndrome due to a mutation in DPAGT1. Neuromuscul Disord 23:469–472
Jackson BJ, Kukuruzinska MA, Robbins P (1993) Biosynthesis of asparagine-linked oligosaccharides in Saccharomyces cerevisiae: the alg2 mutation. Glycobiology 3:357–364
Lu J, Takahashi T, Ohoka A et al (2012) Alg14 organizes the formation of a multiglycosyltransferase complex involved in initiation of lipid-linked oligosaccharide biosynthesis. Glycobiology 22:504–516
Monies DM, Al-Hindi HN, Al-Muhaizea Ma et al (2014) Clinical and pathological heterogeneity of a congenital disorder of glycosylation manifesting as a myasthenic/myopathic syndrome. Neuromuscul Disord 24:353–359
Rodriguez Cruz P, Sewry C, Beeson D et al (2014) Science direct congenital myopathies with secondary neuromuscular transmission defects.A case report and review of the literature. Neuromuscul Disord. doi:10.1016/j.nmd.2014.07.005
Illingworth M, Main M, Pitt M, et al. RYR1-related congenital myopathy with fatigable weakness, responding to pyridostigimine
Munot P, Lashley D, Jungbluth H et al (2010) Congenital fibre type disproportion associated with mutations in the tropomyosin 3 (TPM3) gene mimicking congenital myasthenia. Neuromuscul Disord 20:796–800
Robb Sa, Sewry Ca, Dowling JJ et al (2011) Impaired neuromuscular transmission and response to acetylcholinesterase inhibitors in centronuclear myopathies. Neuromuscul Disord 21:379–386
Liewluck T, Shen X-M, Milone M, Engel AG (2012) Endplate structure and parameters of neuromuscular transmission in sporadic centronuclear myopathy associated with myasthenia. Neuromuscul Disord 21:387–395
Gibbs EM, Clarke NF, Rose K et al (2013) Neuromuscular junction abnormalities in DNM2-related centronuclear myopathy. J Mol Med (Berl) 91:727–737
Servais L, Baudoin H, Zehrouni K et al (2013) Pregnancy in congenital myasthenic syndrome. J Neurol 260:815–819
Lorenzoni PJ, Scola RH, Kay CSK et al (2013) Salbutamol therapy in congenital myasthenic syndrome due to DOK7 mutation. J Neurol Sci 331:155–157
Burke G, Hiscock A, Klein A et al (2013) Salbutamol benefits children with congenital myasthenic syndrome due to DOK7 mutations. Neuromuscul Disord 23:170–175
Lashley D, Palace J, Jayawant S et al (2010) Ephedrine treatment in congenital myasthenic syndrome due to mutations in DOK7. Neurology 74:1517–1523
Sadeh M, Xin-Ming SEA (2012) Beneficial effect of albuterol in CMS with epsilon subunit mutations. Muscle Nerve 44:289–291
Finlayson S, Palace J, Belaya K et al (2013) Clinical features of congenital myasthenic syndrome due to mutations in DPAGT1. J Neurol Neurosurg Psychiatry 84:1119–1125
Liewluck T, Selcen D, Engel AG (2011) Beneficial effects of Albuterol in congenital endplate acetylcholinesterase deficiency and DOK-7 myasthenia. Muscle Nerve 44:789–794
Wargon I, Richard P, Kuntzer T et al (2012) Long-term follow-up of patients with congenital myasthenic syndrome caused by COLQ mutations. Neuromuscul Disord 22:318–324
Mihaylova V, Müller JS, Vilchez JJ et al (2008) Clinical and molecular genetic findings in COLQ-mutant congenital myasthenic syndromes. Brain 131:747–759
Guven A, Demirci M, Anlar B (2012) Recurrent COLQ mutation in congenital myasthenic syndrome. Pediatr Neurol 46:253–256
Duran GS, Uzunhan TA, Ekici B et al (2013) Severe scoliosis in a patient with COLQ mutation and congenital myasthenic syndrome: a clue for diagnosis. Acta Neurol Belg 113:531–532
Bestue-Cardiel M, de Cabezón-Alvarez a Sáenz, Capablo-Liesa JL et al (2005) Congenital endplate acetylcholinesterase deficiency responsive to ephedrine. Neurology 65:144–146
Choi K-R, Berrera M, Reischl M et al (2012) Rapsyn mediates subsynaptic anchoring of PKA type I and stabilisation of acetylcholine receptor in vivo. J Cell Sci 125:714–723
Acknowledgments
We gratefully acknowledge the UK National Specialised Commissioning Team for funding to the Diagnostic and Advisory service for CMS in Oxford.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodríguez Cruz, P.M., Palace, J. & Beeson, D. Inherited disorders of the neuromuscular junction: an update. J Neurol 261, 2234–2243 (2014). https://doi.org/10.1007/s00415-014-7520-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7520-7