Abstract
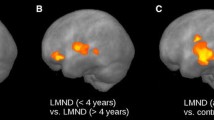
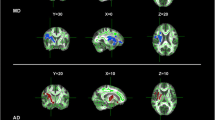
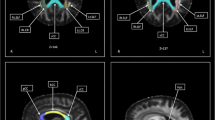
Previous studies using diffusion tensor imaging (DTI) have examined for differences between bulbar- and limb-onset amyotrophic lateral sclerosis (ALS). Findings between studies have been markedly inconsistent, though possibly as a consequence of poor matching for confounding variables. To address this problem, this study contrasted the DTI profiles of limb-onset (ALS-L) and bulbar-onset (ALS-B) in groups that were tightly matched for the potential confounding effects of power, age, cognitive impairment and motor dysfunction. 14 ALS-L and 14 ALS-B patients were selected from a large prospective study so as to be matched on clinical and demographic features. All subjects, including 29 controls, underwent neuropsychological and neurological assessment. Tract-based spatial statistics and region of interest techniques were used to analyse fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD) and axial diffusivity (λ 1). Extensive bilateral FA and RD changes along the corticospinal tract were found in ALS-B compared to controls, p (corrected) <0.05; a similar distribution was seen for ALS-L at a less stringent statistical threshold. ROI analyses also showed more significant changes in ALS-B than ALS-L when each was compared to controls; for FA, MD and RD the changes reached statistical significance in the direct contrast between the two patient groups. With careful matching for confounding factors, the results suggest that ALS-B is associated with greater central white matter degeneration than ALS-L, possibly contributing to the known worse prognosis of ALS-B. The study, however, found no evidence that the spatial distribution of white matter degeneration differs between these groups.


Similar content being viewed by others
References
Roche JC, Rojas-Garcia R, Scott KM, Scotton W, Ellis CE, Burman R, Wijesekera L, Turner MR, Leigh PN, Shaw CE, Al-Chalabi A (2012) A proposed staging system for amyotrophic lateral sclerosis. Brain 135(Pt 3):847–852
Ganesalingam J, Stahl D, Wijesekera L, Galtrey C, Shaw CE, Leigh PN, Al-Chalabi A (2009) Latent cluster analysis of ALS phenotypes identifies prognostically differing groups. PLoS ONE 4(9):e7107
van der Graaff MM, Sage CA, Caan MW, Akkerman EM, Lavini C, Majoie CB, Nederveen AJ, Zwinderman AH, Vos F, Brugman F, van den Berg LH, de Rijk MC, van Doorn PA, Van Hecke W, Peeters RR, Robberecht W, Sunaert S, de Visser M (2011) Upper and extra-motoneuron involvement in early motoneuron disease: a diffusion tensor imaging study. Brain 134(Pt 4):1211–1228
Prell T, Peschel T, Hartung V, Kaufmann J, Klauschies R, Bodammer N, Kollewe K, Dengler R, Grosskreutz J (2013) Diffusion tensor imaging patterns differ in bulbar and limb onset amyotrophic lateral sclerosis. Clin Neurol Neurosurg 115(8):1281–1287
Ellis CM, Simmons A, Jones DK, Bland J, Dawson JM, Horsfield MA, Williams SC, Leigh PN (1999) Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 53(5):1051–1058
Sach M, Winkler G, Glauche V, Liepert J, Heimbach B, Koch MA, Buchel C, Weiller C (2004) Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain 127(Pt 2):340–350
Jones DK, Cercignani M (2010) Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed 23(7):803–820
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage 31(4):1487–1505
Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron D (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Scler Other Motor Neuron Disord 1(5):293–299
Rascovsky K, Hodges JR, Knopman D et al (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134(Pt 9):2456–2477
Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 169(1–2):13–21
Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–699
Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A (2002) Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 47(6):1202–1210
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23(Suppl 1):S208–S219
Jenkinson M, Smith S (2001) A global optimisation method for robust affine registration of brain images. Med Image Anal 5(2):143–156
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17(3):143–155
Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44(1):83–98
Hw L (1967) On the Kolmogorov–Smirnov test for normality with mean and variance unknown. J Am Stat Assoc 62:399–402
Mann HB, Whitney DR (1947) On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat 18:50–60
Muller HP, Unrath A, Huppertz HJ, Ludolph AC, Kassubek J (2012) Neuroanatomical patterns of cerebral white matter involvement in different motor neuron diseases as studied by diffusion tensor imaging analysis. Amyotrophic Lateral Scler 13(3):254–264
Iwata NK, Aoki S, Okabe S, Arai N, Terao Y, Kwak S, Abe O, Kanazawa I, Tsuji S, Ugawa Y (2008) Evaluation of corticospinal tracts in ALS with diffusion tensor MRI and brainstem stimulation. Neurology 70(7):528–532
Graham JM, Papadakis N, Evans J, Widjaja E, Romanowski CA, Paley MN, Wallis LI, Wilkinson ID, Shaw PJ, Griffiths PD (2004) Diffusion tensor imaging for the assessment of upper motor neuron integrity in ALS. Neurology 63(11):2111–2119
Filippini N, Douaud G, Mackay CE, Knight S, Talbot K, Turner MR (2010) Corpus callosum involvement is a consistent feature of amyotrophic lateral sclerosis. Neurology 75(18):1645–1652
Agosta F, Pagani E, Petrolini M, Caputo D, Perini M, Prelle A, Salvi F, Filippi M (2010) Assessment of white matter tract damage in patients with amyotrophic lateral sclerosis: a diffusion tensor MR imaging tractography study. Am J Neuroradiol 31(8):1457–1461
Abe O, Yamada H, Masutani Y, Aoki S, Kunimatsu A, Yamasue H, Fukuda R, Kasai K, Hayashi N, Masumoto T, Mori H, Soma T, Ohtomo K (2004) Amyotrophic lateral sclerosis: diffusion tensor tractography and voxel-based analysis. NMR Biomed 17(6):411–416
Smith MC (1960) Nerve fibre degeneration in the brain in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 23(4):269–282
Acosta-Cabronero J, Alley S, Williams GB, Pengas G, Nestor PJ (2012) Diffusion tensor metrics as biomarkers in Alzheimer’s disease. PLoS ONE 7(11):e49072
Sajjadi SA, Acosta-Cabronero J, Patterson K, Diaz-de-Grenu LZ, Williams GB, Nestor PJ (2013) Diffusion tensor magnetic resonance imaging for single subject diagnosis in neurodegenerative diseases. Brain 136(Pt 7):2253–2261
Acosta-Cabronero J, Williams GB, Pengas G, Nestor PJ (2010) Absolute diffusivities define the landscape of white matter degeneration in Alzheimer’s disease. Brain 133(Pt 2):529–539
Nir TM, Jahanshad N, Villalon-Reina JE, Toga AW, Jack CR, Weiner MW, Thompson PM, Alzheimer’s Disease Neuroimaging I (2013) Effectiveness of regional DTI measures in distinguishing Alzheimer’s disease, MCI, and normal aging. NeuroImage Clin 3:180–195
Cistaro A, Valentini MC, Chio A, Nobili F, Calvo A, Moglia C, Montuschi A, Morbelli S, Salmaso D, Fania P, Carrara G, Pagani M (2012) Brain hypermetabolism in amyotrophic lateral sclerosis: a FDG PET study in ALS of spinal and bulbar onset. Eur J Nucl Med Mol Imaging 39(2):251–259
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standard
The study was approved by the local ethics committee of Otto-von-Guericke University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cardenas-Blanco, A., Machts, J., Acosta-Cabronero, J. et al. Central white matter degeneration in bulbar- and limb-onset amyotrophic lateral sclerosis. J Neurol 261, 1961–1967 (2014). https://doi.org/10.1007/s00415-014-7434-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7434-4