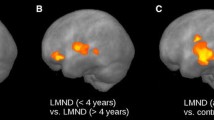
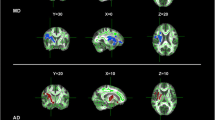
Abstract
Diffusion tensor imaging (DTI) has become a useful tool for investigating early white matter (WM) abnormalities in motor neuron disease. Furthermore, fiber tracking packages that apply multi-tensorial algorithms, such as q-ball imaging (QBI), have been proposed as alternative approaches to overcome DTI limitations in depicting fiber tracts with different orientations within the same voxel. We explored motor and extra-motor WM tract abnormalities in phenotypically heterogeneous amyotrophic lateral sclerosis (ALS) cases aiming to establish a consistent QBI-based WM signature of disease. We performed a whole-brain, QBI tract-based spatial statistics analysis with deterministic tractography of genu, body and splenium of corpus callosum (CC) and corticospinal tracts (CST) in 20 ALS patients (12 classical and 8 lower motor neuron variants) compared to 20 healthy controls. Mean tract length, fiber volume and density, and generalized fractional anisotropy were extracted and related to clinical indices of pyramidal impairment (upper motor neuron score), disease disability (ALS functional rating scale-revised) and progression. ALS patients showed significantly decreased fiber density and volume, and increased tract length in all regions of CC and left CST (p < 0.05, corrected). In CC body, pyramidal impairment was inversely correlated to fiber density (p = 0.01), while in CC splenium, clinical disability (p = 0.01) and progression (p = 0.02) were inversely correlated to tract length. Our findings further suggest that QBI tractography might represent a promising approach for investigating structural alterations in neurodegenerative diseases and confirm that callosal involvement is a consistent feature of most ALS variants, significantly related to both pyramidal dysfunction and disease disability.



Similar content being viewed by others
Abbreviations
- ACE-R:
-
Addenbrooke’s cognitive examination revised
- ALS:
-
Amyotrophic lateral sclerosis
- ALSFRS-R:
-
ALS functional rating scale-revised
- BDNF:
-
Brain-derived neurotrophic factor
- CC:
-
Corpus callosum
- CST:
-
Corticospinal tract
- DTI:
-
Diffusion tensor imaging
- DW-MRI:
-
Diffusion-weighted-magnetic resonance imaging
- FMRIB:
-
Functional magnetic resonance imaging of the brain
- FDT:
-
FMRIB’s diffusion toolbox
- FLIRT:
-
FMRIB’s linear image registration tool
- FrSBe:
-
Frontal systems behavior
- FSL:
-
FMRIB software library
- FUS/TLS:
-
Fused in sarcoma/translocated in liposarcoma
- GFA:
-
Generalized fractional anisotropy
- HARDI:
-
High-angular-resolution diffusion imaging
- IGF 1:
-
Insulin-like growth factor 1
- LMN:
-
Lower motor neuron
- MND:
-
Motor neuron disease
- MNI:
-
Montreal Neurological Institute
- ODF:
-
Orientation distribution function
- PMA:
-
Progressive muscular atrophy
- PLS:
-
Primary lateral sclerosis
- QBI:
-
Q-ball imaging
- SMN1:
-
Survival motor neuron 1
- SOD1:
-
Superoxide dismutase 1
- TARDBP:
-
Transactive response DNA-binding protein
- TBSS:
-
Tract-based spatial statistics
- TCFE:
-
Threshold-free cluster enhancement
- TDP-43:
-
Transactivating DNA binding protein-43
- UMN:
-
Upper motor neuron
- VOI:
-
Volume of interest
- WM:
-
White matter
References
Donaghy M (1999) Classification and clinical features of motor neurone diseases and motor neuropathies in adults. J Neurol 246:331–333
Turner MR, Kiernan MC, Leigh PN, Talbot K (2009) Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol 8:94–109
Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A (2000) In vivo fiber tractography using DT-MRI data. Magn Reson Med 44:625–632
Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed 15:435–455
Turner MR, Modo M (2010) Advances in the application of MRI to amyotrophic lateral sclerosis. Expert Opin Med Diagn 4:483–496
Agosta F, Chiò A, Cosottini M, De Stefano N, Falini A, Mascalchi M, Rocca MA, Silani V, Tedeschi G, Filippi M (2010) The present and the future of neuroimaging in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol 31:1769–1777
Sach M, Winkler G, Glauche V, Liepert J, Heimbach B, Koch MA, Büchel C, Weiller C (2004) Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain 127:340–350
Ciccarelli O, Behrens TE, Johansen-Berg H, Talbot K, Orrell RW, Howard RS, Nunes RG, Miller DH, Matthews PM, Thompson AJ, Smith SM (2009) Investigation of white matter pathology in ALS and PLS using tract-based spatial statistics. Hum Brain Mapp 30:615–624
van der Graaff MM, Sage CA, Caan MW, Akkerman EM, Lavini C, Majoie CB, Nederveen AJ, Zwinderman AH, Vos F, Brugman F, van den Berg LH, de Rijk MC, van Doorn PA, Van Hecke W, Peeters RR, Robberecht W, Sunaert S, de Visser M (2011) Upper and extra-motoneuron involvement in early motoneuron disease: a diffusion tensor imaging study. Brain 134:1211–1228
Müller HP, Unrath A, Huppertz HJ, Ludolph AC, Kassubek J (2012) Neuroanatomical patterns of cerebral white matter involvement in different motor neuron diseases as studied by diffusion tensor imaging analysis. Amyotroph Lateral Scler 13:254–264
Prudlo J, Bißbort C, Glass A, Grossmann A, Hauenstein K, Benecke R, Teipel SJ (2012) White matter pathology in ALS and lower motor neuron ALS variants: a diffusion tensor imaging study using tract-based spatial statistics. J Neurol 259:1848–1859
Agosta F, Galantucci S, Riva N, Chiò A, Messina S, Iannaccone S, Calvo A, Silani V, Copetti M, Falini A, Comi G, Filippi M (2013) Intrahemispheric and interhemispheric structural network abnormalities in PLS and ALS. Hum Brain Mapp doi. doi:10.1002/hbm.22286
Rose S, Pannek K, Bell C, Baumann F, Hutchinson N, Coulthard A, McCombe P, Henderson R (2012) Direct evidence of intra- and interhemispheric corticomotor network degeneration in amyotrophic lateral sclerosis: an automated MRI structural connectivity study. NeuroImage 59:2661–2669
Trojsi F, Corbo D, Caiazzo G, Piccirillo G, Monsurrò MR, Cirillo S, Esposito F, Tedeschi G (2013) Motor and extramotor neurodegeneration in amyotrophic lateral sclerosis: a 3T high angular resolution diffusion imaging (HARDI) study. Amyotroph Lateral Scler Frontotemporal Degener. doi:10.3109/21678421.2013.785569
Tuch DS, Resse TG, Wiegell MR, Wedeen VJ (2003) Diffusion MRI of complex neural architecture. Neuron 40:885–895
Sotiropoulos SN, Bai L, Morgan PS, Constantinescu CS, Tench CR (2010) Brain tractography using Q-ball imaging and graph theory: improved connectivities through fibre crossings via a model-based approach. NeuroImage 49:2444–2456
Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299
Wijesekera LC, Mathers S, Talman P, Galtrey C, Parkinson MH, Ganesalingam J, Willey E, Ampong MA, Ellis CM, Shaw CE, Al-Chalabi A, Leigh PN (2009) Natural history and clinical features of the flail arm and flail leg ALS variants. Neurology 72:1087–1094
Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A, BDNF ALS Study Group (Phase III) (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function: BDNF ALS study group (Phase III). J Neurol Sci 169:13–21
Ellis CM, Simmons A, Jones DK, Bland J, Dawson JM, Horsfield MA, Williams SC, Leigh PN (1999) Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 53:1051–1058
Grace J, Stout JC, Malloy PF (1999) Assessing frontal lobe behavioral syndromes with the frontal lobe personality scale. Assessment 6:269–284
Turner MR, Cagnin A, Turkheimer FE, Miller CC, Shaw CE, Brooks DJ, Leigh PN, Banati RB (2004) Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [(11)C](R)-PK11195 positron emission tomography study. Neurobiol Dis 15:601–609
Filippini N, Douaud G, Mackay CE, Knight S, Talbot K, Turner MR (2010) Corpus callosum involvement is a consistent feature of amyotrophic lateral sclerosis. Neurology 75:1645–1652
Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR (2006) The Addenbrooke’s cognitive examination revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry 21:1078–1085
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage 31:1487–1505
Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S (2008) Tract probability maps in stereotaxic spaces: analysis of white matter anatomy and tract-specific quantification. NeuroImage 39:336–347
Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S (2007) Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage 36:630–644
Abrahams S, Goldstein LH, Suckling J, Ng V, Simmons A, Chitnis X, Atkins L, Williams SC, Leigh PN (2005) Frontotemporal white matter changes in amyotrophic lateral sclerosis. J Neurol 252:321–331
Agosta F, Pagani E, Petrolini M, Caputo D, Perini M, Prelle A, Salvi F, Filippi M (2010) Assessment of white matter tract damage in patients with amyotrophic lateral sclerosis: a diffusion tensor MR imaging tractography study. AJNR Am J Neuroradiol 31:1457–1461
Cirillo M, Esposito F, Tedeschi G, Caiazzo G, Sagnelli A, Piccirillo G, Conforti R, Tortora F, Monsurrò MR, Cirillo S, Trojsi F (2012) Widespread microstructural white matter involvement in amyotrophic lateral sclerosis: a whole-brain DTI study. AJNR Am J Neuroradiol 33:1102–1108
Yamada K, Sakai K, Hoogenraad FG, Holthuizen R, Akazawa K, Ito H, Oouchi H, Matsushima S, Kubota T, Sasajima H, Mineura K, Nishimura T (2007) Multitensor tractography enables better depiction of motor pathways: initial clinical experience using diffusion-weighted MR imaging with standard b-value. AJNR Am J Neuroradiol 9:1668–1673
Wahl M, Strominger Z, Jeremy RJ, Barkovich AJ, Wakahiro M, Sherr EH, Mukherjee P (2009) Variability of homotopic and heterotopic callosal connectivity in partial agenesis of the corpus callosum: a 3T diffusion tensor imaging and Q-ball tractography study. AJNR Am J Neuroradiol 30:282–289
Wahl M, Barkovich AJ, Mukherjee P (2010) Diffusion imaging and tractography of congenital brain malformations. Pediatr Radiol 40:59–67
Kuhnt D, Bauer MH, Egger J, Richter M, Kapur T, Sommer J, Merhof D, Nimsky C (2013) Fiber tractography based on diffusion tensor imaging compared with high-angular-resolution diffusion imaging with compressed sensing: initial experience. Neurosurgery 72(Suppl 1):165–175
Jeong JW, Chugani DC, Behen ME, Tiwari VN, Chugani HT (2012) Altered white matter structure of the dentatorubrothalamic pathway in children with autistic spectrum disorders. Cerebellum 11:957–971
Pontabry J, Rousseau F, Oubel E, Studholme C, Koob M, Dietemann JL (2013) Probabilistic tractography using Q-ball imaging and particle filtering: application to adult and in utero fetal brain studies. Med Image Anal 17:297–310
Behrens TEJ, Johansen Berg H, Jbabdi S, Rushworth MFS, Woolrich MW (2007) Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? NeuroImage 34:144–155
Chao YP, Cho KH, Yeh CH, Chou KH, Chen JH, Lin CP (2009) Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography. Hum Brain Mapp 30:3172–3187
Tovar-Moll F, Moll J, de Oliveira-Souza R, Bramati I, Andreiuolo PA, Lent R (2007) Neuroplasticity in human callosal dysgenesis: a diffusion tensor imaging study. Cereb Cortex 17:531–541
Tuch DS (2004) Q-ball imaging. Magn Reson Med 52:1358–1371
Fritzsche KH, Laun FB, Meinzer HP, Stieltjes B (2010) Opportunities and pitfalls in the quantification of fiber integrity: what can we gain from Q-ball imaging? NeuroImage 51:242–251
Graham JM, Papadakis N, Evans J, Widjaja E, Romanowski CA, Paley MN, Wallis LI, Wilkinson ID, Shaw PJ, Griffiths PD (2004) Diffusion tensor imaging for the assessment of upper motor neuron integrity in ALS. Neurology 63:2111–2119
Sage CA, Van Hecke W, Peeters RR, Sijbers J, Robberecht W, Parizel P, Marchal G, Leemans A, Sunaert S (2009) Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis: revisited. Hum Brain Mapp 30:3657–3675
Senda J, Ito M, Watanabe H, Atsuta N, Kawai Y, Katsuno M, Tanaka F, Naganawa S, Fukatsu H, Sobue G (2009) Correlation between pyramidal tract degeneration and widespread white matter involvement in amyotrophic lateral sclerosis: a study with tractography and diffusion tensor imaging. Amyotroph Lateral Scler 10:288–294
Smith MC (1960) Nerve fibre degeneration in the brain in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 23:269–282
Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, Suh E, Van Deerlin VM, Wood EM, Baek Y, Kwong L, Lee EB, Elman L, McCluskey L, Fang L, Feldengut S, Ludolph AC, Lee VM, Braak H, Trojanowski JQ (2013) Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol doi. doi:10.1002/ana.23937
Wang S, Poptani H, Woo JH, Desiderio LM, Elman LB, McCluskey LF, Krejza J, Melhem ER (2006) Amyotrophic lateral sclerosis: diffusion tensor and chemical shift MR imaging at 3.0 T. Radiology 239:831–838
Nihei K, McKee AC, Kowall NW (1993) Patterns of neuronal degeneration in the motor cortex of amyotrophic lateral sclerosis patients. Acta Neuropathol (Berl) 86:55–64
Sasaki S, Iwata M (1999) Ultrastructural change of synapses of Betz cells in patients with amyotrophic lateral sclerosis. Neurosci Lett 268:29–32
Sasaki S, Iwata M (2000) Immunocytochemical and ultrastructural study of the motor cortex in patients with lower motor neuron disease. Neurosci Lett 281:45–48
Vucic S, Nicholson GA, Kiernan MC (2008) Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain 131:1540–1550
Brooks BR, Bushara K, Khan AL, Hershberger J, Wheat JO, Belden D, Henningsen H (2000) Functional magnetic resonance imaging (fMRI) clinical studies in ALS-paradigms, problems and promises. Amyotroph Lateral Scler Other Motor Neuron Disord 1:S23–S32
Turner MR, Leigh PN (2000) Positron emission tomography (PET)-its potential to provide surrogate markers in ALS. Amyotroph Lateral Scler Other Motor Neuron Disord 1:S17–S22
Eisen A (2009) Amyotrophic lateral sclerosis: evolutionary and other perspectives. Muscle Nerve 40:297–304
Billeci L, Calderoni S, Tosetti M, Catani M, Muratori F (2012) White matter connectivity in children with autism spectrum disorders: a tract-based spatial statistics study. BMC Neurology 12:148
Kumar A, Sundaram SK, Sivaswamy L, Behen ME, Makki MI, Ager J, Janisse J, Chugani HT, Chugani DC (2010) Alterations in frontal lobe tracts and corpus callosum in young children with autism spectrum disorder. Cereb Cortex 20:2103–2113
Simard AR, Rivest S (2007) Neuroprotective effects of resident microglia following acute brain injury. J Comp Neurol 504:716–729
Evans MC, Couch Y, Sibson N, Turner MR (2013) Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Mol Cell Neurosci 53:34–41
Bihel E, Roussel S, Toutain J, Bernaudin M, Touzani O (2011) Diffusion tensor MRI reveals chronic alterations in white matter despite the absence of a visible ischemic lesion on conventional MRI: a nonhuman primate study. Stroke 42:1412–1419
Turner MR, Wicks P, Brownstein CA, Massagli MP, Toronjo M, Talbot K, Al-Chalabi A (2011) Concordance between site of onset and limb dominance in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 82:853–854
Krampfl K, Petri S, Gotz F, Mohammadi B, Bufler J (2003) Amyotrophic lateral sclerosis (ALS) and mirror movements in a patient with polymicrogyria. Amyotroph Lateral Scler Other Motor Neuron Disord 4:266–269
Karandreas N, Papaopoulou M, Kokotis P, Papapostolou A, Tsivgoulis G, Zambelis T (2007) Impaired interhemispheric inhibition in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 8:112–118
Corbo D, Caiazzo G, Trojsi F, Monsurrò MR, Gallo A, Bonavita S, Tedeschi G, Esposito F (2013) Advantages of QBI in TBSS analyses. Magn Reson Imaging (in press)
Kuhnt D, Bauer MHA, Sommer J, Merhof D, Nimsky C (2013) Optic radiation fiber tractography in glioma patients based on high angular resolution diffusion imaging with compressed sensing compared with diffusion tensor imaging—initial experience. PLoS ONE 8:e70973
Acknowledgments
We thank Dr. Antonella Paccone for her expert technical support.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
G. Caiazzo and D. Corbo have equally contributed to the manuscript.
Rights and permissions
About this article
Cite this article
Caiazzo, G., Corbo, D., Trojsi, F. et al. Distributed corpus callosum involvement in amyotrophic lateral sclerosis: a deterministic tractography study using q-ball imaging. J Neurol 261, 27–36 (2014). https://doi.org/10.1007/s00415-013-7144-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-013-7144-3