Abstract
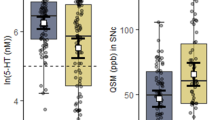
The neurochemical abnormalities underlying vascular parkinsonism (VP) have not been well characterised. A better understanding may help to optimize pharmacological interventions. Since VP patients generally have a poorer response to l-Dopa than Parkinson’s disease (PD) patients, we investigated whether levels of relevant CSF neurotransmitter metabolites may be differentially altered in VP and PD and assessed the potential of neurotransmitter metabolites as biomarkers. We compared CSF levels of homovanillic acid (HVA), 5-hydroxyindolacetic acid, and 3-methoxy-4-hydroxyphenylethyleneglycol, in 16 VP patients, 57 PD patients and 60 non-neurological controls. We found that levels of HVA were reduced in PD compared with both VP and controls but did not differ significantly between VP and controls indicating that dopamine deficiency was less pronounced in VP.
Similar content being viewed by others
References
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Zijlmans JCM, Daniel SE, Hughes AJ, Révész T, Lees AJ (2004) Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov Disord 19:630–640
Aerts MB, Esselink RAJ, Post B, van de Warrenburg BPC, Bloem BR (2012) Improving the diagnostic accuracy in parkinsonism: a three-pronged approach. Pract Neurol 12:77–87
Antonini A, Vitale C, Barone P, Cilia R, Righini A, Bonuccelli U et al (2012) The relationship between cerebral vascular disease and parkinsonism: the VADO study. Parkinsonism Rel Disord 18:775–780
Chang CM, Yu YL, Ng HK, Leung SY, Fong KY (1992) Vascular pseudoparkinsonism. Acta Neurol Scand 86:588–592
Zijlmans JCM, Thijssen HOM, Vogels OJM, Kremer HPHMP, Poels PJE, Schoonderwaldt HC et al (1995) MRI in patients with suspected vascular parkinsonism. Neurology 45:2183–2188
van Zagten M, Lodder J, Kessels F (1998) Gait disorder and parkinsonian signs in patients with stroke related to small deep infarcts and white matter lesions. Mov Disord 13:89–95
Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E et al (2009) Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol 8:1128–1139
Halliday GM, Blumbergs PC, Cotton RGH, Blessing WW, Geffen LB (1990) Loss of brainstem serotonin- and substance P-containing neurons in Parkinson’s disease. Brain Res 510:104–107
Remy P, Doder M, Lees A, Turjanski N, Brooks D (2005) Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 128:1314–1322
Constantinescu R, Richard I, Kurlan R (2007) Levodopa responsiveness in disorders with parkinsonism: a review of the literature. Mov Disord 22:2141–2148
Gupta D, Kuruvilla A (2011) Vascular parkinsonism: what makes it different? Postgrad Med J 87:829–836
Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ (2002) The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 125:861–870
Winikates J, Jankovic J (1999) Clinical correlates of vascular parkinsonism. Arch Neurol 56:98–102
Critchely M (1929) Arteriosclerotic parkinsonism. Brain 52:23–83
FitzGerald PM, Jankovic J (1989) Lower body parkinsonism: evidence for vascular etiology. Mov Disord 4:249–260
Hoehn MMMD, Yahr MDMD (2001) Parkinsonism: onset, progression, and mortality. Neurology 57(10):S11–S26
Fahn S, Elton RL (1987) Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB (eds) Recent developments in Parkinson’s Disease. Macmillan Healthcare Information, Florham Park, pp 153–163
Brautigam C, Wevers RA, Jansen RJ, Smeitink JA, de Rijk-van Andel JF, Gabreels FJ, Hoffmann GF (1998) Biochemical hallmarks of tyrosine hydroxylase deficiency. Clin Chem 44:1897–1904
Abdo WF, van de Warrenburg BPC, Munneke M, van Geel WJA, Bloem BR, Kremer HPH et al (2006) CSF analysis differentiates multiple-system atrophy from idiopathic late-onset cerebellar ataxia. Neurology 67:474–479
Tohgi H, Abe T, Saheki M, Yamazaki K, Murata T (1997) Concentration of catecholamines and indoleamines in the cerebrospinal fluid of patients with vascular parkinsonism compared to Parkinson’s disease patients. J Neural Transm 104:441–449
Espino A, Ambrosio S, Bartrons R, Bendahan G, Calopa M (1994) Cerebrospinal monoamine metabolites and amino acid content in patients with parkinsonian syndrome and rats lesioned with MPP+. J Neural Transm 7:167–176
Hartikainen P, Soininen H, Reinikainen KJ, Sirviö J, Soikkeli R, Riekkinen PJ (1991) Neurotransmitter markers in the cerebrospinal fluid of normal subjects effects of aging and other confounding factors. J Neural Transm 84(1–2):103–117
Nilsson C, Stahlberg F, Thomsen C, Henriksen O, Herning M, Owman C (1992) Circadian variation in human cerebrospinal fluid production measured by magnetic resonance imaging. Am J Physiol Regul Integr Comp Physiol 262(1):R20–R24
de Laat KF, van Norden AGW, Gons RAR, van Uden IWM, Zwiers MP, Bloem BR et al (2012) Cerebral white matter lesions and lacunar infarcts contribute to the presence of mild parkinsonian signs. Stroke 43:2574–2579
Eisenhofer G, Kopin IJ, Goldstein DS (2004) Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 56:331–349
Zijlmans JCM, Katzenschlager R, Daniel SE, Lees AJL (2004) The l-Dopa response in vascular parkinsonism. J Neurol Neurosurg Psychiatry 75:545–547
Glass PG, Lees AJ, Bacellar A, Zijlmans J, Katzenschlager R, Silveira-Moriyama L (2012) The clinical features of pathologically confirmed vascular parkinsonism. J Neurol Neurosurg Psychiatry 83:1027–1029
Kish SJ, Tong J, Hornykiewicz O, Rajput A, Chang L-J, Guttman M et al (2008) Preferential loss of serotonin markers in caudate versus putamen in Parkinson’s disease. Brain 131:120–131
Reed MC, Best JA, Nijhout HF (2012) Mathematical insights into the effects of levodopa. Front Integr Neurosci 6:21
Navailles S, De Deurwaerdere P (2012) Contribution of serotonergic transmission to the motor and cognitive effects of high-frequency stimulation of the subthalamic nucleus or levodopa in Parkinson’s disease. Mol Neurobiol 45:173–185
Acknowledgments
We thank the technicians of the Department of Laboratory Medicine (LGEM) for CSF analysis.
Disclosures
Megan Herbert reports no disclosures.
Bea Kuiperij reports no disclosures.
Bastiaan Bloem received honoraria from serving on the scientific advisory board for Boehringer Ingelheim, Teva, Glaxo-Smith-Kline and Novartis, and received research support from the Netherlands Organization for Scientific Research, the Michael J Fox Foundation, the Prinses Beatrix Foundation, the Stichting Internationaal Parkinson Fonds and the Alkemade Keuls fonds.
Marcel Verbeek receives funding from Joint Programming Neurodegenerative Disease, Alzheimer’s Drug Discovery Foundation, American Alzheimer Association, Van Alkemade Keuls fonds and Center for Translational Molecular Medicine.
Conflicts of interest
The authors declare no conflicts of interest in relation to this study.
Ethical standard
Samples selected from our CSF biobank databases were collected for routine purposes according to standard protocols. All subjects gave their informed consent at the time of lumbar puncture including later use for scientific purposes. CSF sampling was performed in accordance with the research protocols approved by the institutional review board in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
415_2013_7126_MOESM2_ESM.tif
Figure S1. Levels of neurotransmitters metabolites in CSF. CSF 5-HIAA (A) and HVA (B) levels were higher in VP patients compared with PD patients but no differences in MHPG levels (C) were observed (TIFF 695 kb)
Rights and permissions
About this article
Cite this article
Herbert, M.K., Kuiperij, H.B., Bloem, B.R. et al. Levels of HVA, 5-HIAA, and MHPG in the CSF of vascular parkinsonism compared to Parkinson’s disease and controls. J Neurol 260, 3129–3133 (2013). https://doi.org/10.1007/s00415-013-7126-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-013-7126-5