Abstract
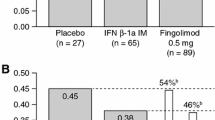
In the 12-month phase 3 TRANSFORMS study, fingolimod showed greater efficacy than intramuscular interferon beta (IFNβ)-1a in patients with relapsing–remitting multiple sclerosis (RRMS). This study analyzed fingolimod efficacy compared with IFNβ-1a in patient subgroups from TRANSFORMS. Patients were randomized to receive fingolimod or weekly IM IFNβ-1a for 12 months. Analyses of efficacy included annualized relapse rate (ARR), and magnetic resonance imaging (MRI) measures [gadolinium (Gd)-enhancing T1 lesions, new/newly enlarged (active) T2 lesions, brain volume change]. Subgroups were defined based on demographics, disease characteristics (baseline EDSS score, relapse rate, and MRI parameters), and response to previous therapy. Fingolimod 0.5 mg reduced ARR over 12 months by 32–59 % relative to IFNβ-1a in all subgroups defined by demographic factors or baseline disease characteristics. Fingolimod also reduced the number of new Gd-enhancing lesions, active T2 lesions, and the rate of brain volume loss, versus IFNβ-1a in most (95 %) subgroups. In patients with high disease activity despite IFNβ treatment in the year before study, fingolimod 0.5 mg reduced ARR by 61 % relative to IFNβ-1a. Reductions in lesion counts and brain volume loss also favored fingolimod in these patients. In conclusion, consistently better efficacy was observed for fingolimod compared with IFNβ-1a across different subgroups of patients with RRMS.





Similar content being viewed by others
References
Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Kappos L (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362:402–415
Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362(5):387–401
Khatri B, Barkhof F, Comi G, Hartung HP, Kappos L, Montalban X, Pelletier J, Stites T, Wu S, Holdbrook F, Zhang-Auberson L, Francis G, Cohen JA (2011) Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol 10(6):520–529
Brex PA, Ciccarelli O, O’Riordan JI, Sailer M, Thompson AJ, Miller DH (2002) A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med 346(3):158–164
Confavreux C, Vukusic S (2006) Age at disability milestones in multiple sclerosis. Brain 129(Pt 3):595–605
Confavreux C, Vukusic S, Adeleine P (2003) Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain 126(Pt 4):770–782
Losseff NA, Miller DH, Kidd D, Thompson AJ (2001) The predictive value of gadolinium enhancement for long term disability in relapsing-remitting multiple sclerosis—preliminary results. Mult Scler 7(1):23–25
Scott TF, Schramke CJ, Novero J, Chieffe C (2000) Short-term prognosis in early relapsing-remitting multiple sclerosis. Neurology 55(5):689–693
Devonshire V, Havrdova E, Radue EW, O’Connor P, Zhang-Auberson L, Agoropoulou C, Haring DA, Francis G, Kappos L (2012) Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double-blind, randomised, placebo-controlled FREEDOMS study. Lancet Neurol 11(5):420–428
Rothman KJ (1990) No adjustments are needed for multiple comparisons. Epidemiology 1(1):43–46
Tremlett H, Zhao Y, Joseph J, Devonshire V (2008) Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry 79(12):1368–1374
Portaccio E, Zipoli V, Siracusa G, Sorbi S, Amato MP (2008) Long-term adherence to interferon beta therapy in relapsing-remitting multiple sclerosis. Eur Neurol 59(3–4):131–135
Zwibel HL (2006) Glatiramer acetate in treatment-naive and prior interferon-beta-1b-treated multiple sclerosis patients. Acta Neurol Scand 113(6):378–386
Jacobs LD, Beck RW, Simon JH, Kinkel RP, Brownscheidle CM, Murray TJ, Simonian NA, Slasor PJ, Sandrock AW (2000) Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med 343(13):898–904
Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, Fischer JS, Goodkin DE, Granger CV, Simon JH, Alam JJ, Bartoszak DM, Bourdette DN, Braiman J, Brownscheidle CM, Coats ME, Cohan SL, Dougherty DS, Kinkel RP, Mass MK, Munschauer FE 3rd, Priore RL, Pullicino PM, Scherokman BJ, Whitham RH et al (1996) Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 39(3):285–294
Li DK, Paty DW (1999) Magnetic resonance imaging results of the PRISMS trial: a randomized, double-blind, placebo-controlled study of interferon-beta1a in relapsing-remitting multiple sclerosis. Prevention of relapses and disability by interferon-beta1a subcutaneously in multiple sclerosis. Ann Neurol 46(2):197–206
PRISMS study group (1998) Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (prevention of relapses and disability by interferon beta-1a subcutaneously in multiple sclerosis) Study Group. Lancet 352(9139):1498–1504
Acknowledgments
The authors thank the patients who participated in the study, the study site personnel, and C. Brechin PhD (Oxford PharmaGenesis™ Ltd) and H. Salloukh (Novartis Pharma AG) for editorial assistance. All authors had full access to all data in the study, participated in writing the manuscript, and approved the full manuscript before the corresponding author took final responsibility for the decision to submit for publication. This study was funded by Novartis Pharma AG, Basel, Switzerland.
Conflicts of interest
Prof. Cohen has received reimbursement for travel or consulting from Biogen Idec, Elan, Novartis, Teva, and Vaccinex. He has also received research support paid to his institution from Biogen Idec, Novartis, Receptos, Teva, Synthon, and Vaccinex. Dr. Barkhof has received consultancy fees from Bayer Schering Pharma, Sanofi-Aventis, Biogen Idec, GE Medical Systems, Genzyme, Synthon, Novartis and Roche. He has served as an editorial board member for Brain, Journal of Neurology, European Radiology and Neuroradiology. He has received payment for speaker services from Novartis and Serono. Barkhof has also received research support from the Dutch Foundation for MS Research. Prof. Comi has received consulting fees for speaker services from Novartis, Teva Pharmaceutical Industries, Sanofi-Aventis, Merck Serono, Bayer and Actelion. He has also received lecture fees from Novartis, Teva Pharmaceutical Industries, Sanofi-Aventis, Merck Serono, Biogen Dompè, Bayer and Serono Symposia International Foundation. Prof. Izquierdo has received payment for board membership of Biogen, Novartis, Sanofi, Serono and Teva. He has also received consultancy fees or honorarium from Biogen Idec, Merck, Bayern, Sanofi, Teva and Novartis. Dr. Khatri has received consultancy fees from Teva, Biogen Idec, Bayer, Pfizer, Genzyme, Questcor, Avanit, Novartis and Teurimo BCT. He has received research support from Biogen Idec and Novartis. Dr. Montalban has received speaking honoraria and travel expenses for scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with Bayer Schering Pharma, Biogen Idec, EMD Merck Serono, Genentech, Genzyme, Novartis, Sanofi-Aventis, Teva Pharmaceutical Industries and Almirall. Prof. Pelletier has received payment for scientific advisory boards for Allergan, Bayer Schering Pharma, Biogen Idec, Genzyme, Merck Serono, Novartis, Sanofi and Teva. He has received non-profit foundation support from ARSEP, academic research support from PHRC, and unconditional research support from Bayer Schering Pharma, Biogen Idec, Merck Serono, Novartis, Sanofi and Teva. Dr. Eckert is an employee of Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA. Dr. Häring is an employee of Novartis Pharma AG, Basel, Switzerland. Dr. Francis is an employee of Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the TRANSFORMS Study Group.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cohen, J.A., Barkhof, F., Comi, G. et al. Fingolimod versus intramuscular interferon in patient subgroups from TRANSFORMS. J Neurol 260, 2023–2032 (2013). https://doi.org/10.1007/s00415-013-6932-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-013-6932-0