Abstract
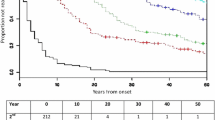
The aim of the study was to estimate the rate of conversion from clinically isolated syndrome (CIS) to multiple sclerosis (MS) and to investigate variables predicting conversion in a cohort of patients presenting with symptoms suggestive of MS. Patients with a first symptom suggestive of MS in the preceding 6 months and exclusion of other diseases were enrolled in an observational prospective study from December 2004 through June 2007. Conversion from CIS to MS according to both McDonald and Clinically Defined Multiple Sclerosis (CDMS) criteria was prospectively recorded until March 2010. The multivariate Cox proportional hazard model was used to assess the best predictive factors of conversion from CIS to MS. Among 168 patients included in the analysis, 122 converted to MS according to McDonald criteria whereas 81 converted to MS according to CDMS criteria. The 2-year probability of conversion was 57 % for McDonald Criteria and 36 % for CDMS criteria. Variables at enrolment significantly associated with conversion according to McDonald criteria were age and positivity for Barkhof criteria, and according to Poser’s CDMS criteria, age, positivity for Barkhof criteria and no disease modifying therapy. In this large prospective cohort study the conversion rate from CIS to MS in patients presenting with recent symptoms suggestive of MS was within the range of previous observational studies and lower than that reported in the placebo arm of randomized trials. We confirm the prognostic value of MRI in addition to the previous experimental data on the protective role of disease-modifying therapies.


Similar content being viewed by others
References
Sastre-Garriga J, Tintore M, Rovira A et al (2003) Conversion to multiple sclerosis after a clinically isolated syndrome of the brainstem: cranial magnetic resonance imaging, cerebrospinal fluid and neurophysiological findings. Mult Scler 9(1):39–43
Miller D, Barkhof F, Montalban X et al (2005) Clinically isolated syndromes suggestive of multiple sclerosis, part I: natural history, pathogenesis, diagnosis, and prognosis. Lancet Neurol 4(5):281–288
Beck RW, Chandler DL, Cole SR et al (2002) Interferon beta-1a for early multiple sclerosis: CHAMPS trial subgroup analyses. Ann Neurol 51(4):481–490
Poser CM, Paty DW, Scheinberg L et al (1983) New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 13(3):227–231
Tintore M, Rovira A, Arrambide G et al (2010) Brainstem lesions in clinically isolated syndromes. Neurology 75(21):1933–1938
Paolino E, Fainardi E, Ruppi P et al (1996) A prospective study on the predictive value of CSF oligoclonal bands and MRI in acute isolated neurological syndromes for subsequent progression to multiple sclerosis. J Neurol Neurosurg Psychiatry 60(5):572–575
Sormani MP, Tintore M, Rovaris M et al (2008) Will Rogers phenomenon in multiple sclerosis. Ann Neurol 64(4):428–433
Morrissey SP, Miller DH, Kendall BE et al (1993) The significance of brain magnetic resonance imaging abnormalities at presentation with clinically isolated syndromes suggestive of multiple sclerosis. A 5-year follow-up study. Brain 116(Pt 1):135–146
Comi G, Filippi M, Barkhof F et al (2001) Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet 357(9268):1576–1582
Kappos L, Polman CH, Freedman MS et al (2006) Treatment with interferon β-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 67(7):1242–1249
Comi G, De Stefano N, Freedman MS, et al (2012) Comparison of two dosing frequencies of subcutaneous interferon beta-1a in patients with a first clinical demyelinating event suggestive of multiple sclerosis (REFLEX): a phase 3 randomised controlled trial. Lancet Neurol 11(1):33–41
Comi G, Martinelli V, Rodegher M et al (2009) Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet 374(9700):1503–1511
Rojas JI, Patrucco L, Cristiano E (2010) Oligoclonal bands and MRI in Clinically Isolated Syndromes: predicting conversion time to multiple sclerosis. J Neurol 257(7):1188–1191
Korteweg T, Tintore M, Uitdehaag B et al (2006) MRI criteria for dissemination in space in patients with clinically isolated syndromes: a multicentre follow-up study. Lancet Neurol 5(3):221–227
Chard DT, Dalton CM, Swanton J et al (2011) MRI only conversion to multiple sclerosis following a clinically isolated syndrome. J Neurol Neurosurg Psychiatry 82(2):176–179
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33(11):1444–1452
Fischer JS, Rudick RA, Cutter GR et al (1999) The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler 5(4):244–250
Solari A, Radice D, Manneschi L et al (2005) The multiple sclerosis functional composite: different practice effects in the three test components. J Neurol Sci 228(1):71–74
Kalkers NF, Bergers L, de Groot V et al (2001) Concurrent validity of the MS Functional Composite using MRI as a biological disease marker. Neurology 56(2):215–219
McDonald WI, Compston A, Edan G et al (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 50(1):121–127
Polman CH, Reingold SC, Edan G et al (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 58(6):840–846
Barkhof F, Filippi M, Miller DH et al (1997) Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 120(Pt 11):2059–2069
Tintore M, Rovira A, Martinez MJ et al (2000) Isolated demyelinating syndromes: comparison of different MR imaging criteria to predict conversion to clinically definite multiple sclerosis. AJNR Am J Neuroradiol 21(4):702–706
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302
Rocca MA, Agosta F, Sormani MP et al (2008) A three-year, multi-parametric MRI study in patients at presentation with CIS. J Neurol 255(5):683–691
McAlpine D (1972) Multiple sclerosis: a re-appraisal. In: Acheson ED (ed) McAlpine D LC. The problem of diagnosis. Churchill Livingstone, Edinburgh, pp 142–178
Tintoré M, Rovira A, Río J (2006) Baseline MRI predicts future attacks and disability in clinically isolated syndromes. Neurology 67:968–972
Feuillet L, Reuter F, Audoin B et al (2007) Early cognitive impairment in patients with clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler 13(1):124–127
Mattarozzi K, Vignatelli L, Baldin E et al (2012) Effect of the disclosure of MS diagnosis on anxiety, mood and quality of life of patients: a prospective study. Int J Clin Pract 66(5):504–514
Acknowledgments
This study was supported with an unconditional grant by Biogen Idec and by Department of Neurological Sciences of the University of Bologna. None of the authors received fees for the participation to the study. Authors have ownership of the data. The study was recognized as “no profit” by all ethical committees to whom it was submitted. We thank Yghea CRO for the precious help.
Conflicts of interest
Prof. Roberto D’Alessandro reports no disclosure. Dr. Luca Vignatelli reports no disclosure. Prof. Alessandra Lugaresi is a Biogen Dompé, Merck Serono and Bayer Schering Advisory Board Member. She received travel grants and honoraria from Bayer Schering, Biogen Dompé, Merck Serono, Novartis, Sanofi Aventis and Teva and research grants from Bayer Schering, Biogen Dompé, Merck Serono, Novartis and Sanofi Aventis. She has also received travel and research grants from the Associazione Italiana Sclerosi Multipla and is a Consultant of “Fondazione Cesare Serono”. Dr. Franco Granella received speaking honoraria from Biogen Idec and travel grants from Biogen Idec, Merck Serono, Sanofi Aventis and Novartis. He received research support from Biogen Idec and Merck Serono. Dr. Elisa Baldin reports no disclosure. Prof. Maria-Rosalia Tola received research funding from Sanofi-Aventis and compensation for consultancy or speaking from Biogen, Sanofi-Aventis, Merk serono, Novartis, Sanofi-Aventis. Dr. Susanna Malagù received travel grants from Bayer Schering, Biogen Dompé, Merck Serono, Novartis, Sanofi Aventis and Teva, honoraria from Bayer Schering and Biogen Dompè and research grants from “Fondazione Cesare Serono”. Dr. Luisa Motti reports no disclosure. Dr. Walter Neri reports no disclosure. Dr. Galeotti reports no disclosure. Dr. Mario Santangelo reports no disclosure. Dr. Laila Fiorani has received travel grants from Bayer Schering, Biogen Dompé, Merck Serono, Novartis, Sanofi Aventis. Dr. Enrico Montanari reports no disclosure. Dr. Cinzia Scandellari reports no disclosure. Dr. Maria Donata Benedetti reports no disclosure. Dr. Maurizio Leone reports no disclosure.
Ethical standard
In the paper it is well reported that the study was approved by all ethical committees.
Author information
Authors and Affiliations
Corresponding author
Additional information
For the GERONIMUS Study Group (members of this study group are listed in "Appendix").
Appendix: GERONIMUS Coinvestigators
Appendix: GERONIMUS Coinvestigators
Katia Mattarozzi, PhD (Dipartimento di Psicologia, Università di Bologna, Psycologic testing coordination); Marianna Pattini, MD (Centro Sclerosi Multipla, U.O di Neurologia, Ospedale Civile –Località Vaio, Site investigators); Caterina Senesi, MD (Centro Sclerosi Multipla Dipartimento di Neuroscienze,Università di Parma, site investigator); Concetta Feo, (U.O.S Neurofisiologia, U.O. Neurologia, Ospedale Santa Maria Nuova, site investigator); Sergio Stecchi, MD; Lucia La Mola; Renzo Balugani, MD (UOSD Riabilitazione-Sclerosi Multipla, Villa Mazzacorati, IRCCS Istituto delle Scienze Neurologiche, Bologna, site investigators); Fabio Pizza, MD (Dipartimento di Scienze Neurologiche, Università di Bologna, site investigator); Laura Delaj, MD; Silvia De Pasqua, MD; Rita Rinaldi, MD (U.O Neurologia, Dipartimento Neuro-Senso-Motorio, Policlinico S.Orsola-Malpighi, Bologna, site investigators); Eleonora Baldi, MD, Luisa Caniatti, MD, Paola Milani, Psychologist (Dipartimento di Neuroscienze,Azienda Ospedaliero Universitaria S. Anna, Ferrara, site investigators and psychologic testing); Vittoria Mussuto, MD; Monica Manzoni, Psycologist (U.O. Neurologia, Ospedale Santa Maria della Scaletta, Imola, site investigators); Mario Casmiro, MD; Claudia Guerrini (Ospedale per gli Infermi,AUSL Ravenna, Faenza, site investigators); Laura Mambelli (U.O. di Neurologia, Presidio Ospedaliero G.B. Morgagni-L. Pierantoni, Forlì, site investigator); Paola Naldi, MD; Laura Collimedaglia, Psychologist (SCDU Neurologia, A.O.U. “Maggiore della Carità”, Novara, site investigators); Erika Pietrolongo, PhD; Valeria Di Tommaso, MD; Giovanna DeLuca, MD; Daniela Travaglini, MD (Centro Sclerosi Multipla, Clinica Neurologica, Dipartimento di Neuroscienze e Imaging, Università “G. d’Annunzio”, Chieti, site investigators and psychological testing); Francesca Rossi, MD; Alberto Gajofatto, MD (Unità Operativa Complessa di Neurologia, Borgo Roma, Verona, site investigators); Marco Pastore Trossello, MD; Luca Faccioli, MD; Luca Spinardi, MD (SSD Neuroradiologia, AOSP, Bologna, MRI review).
Rights and permissions
About this article
Cite this article
D’Alessandro, R., Vignatelli, L., Lugaresi, A. et al. Risk of multiple sclerosis following clinically isolated syndrome: a 4-year prospective study. J Neurol 260, 1583–1593 (2013). https://doi.org/10.1007/s00415-013-6838-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-013-6838-x