Abstract
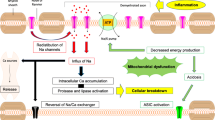
Energy failure is an emerging concept in multiple sclerosis research. Pathological studies have indicated that axonal modifications in response to demyelination may increase neuronal energy demand. At the same time, soluble mediators of inflammation may impair mitochondrial function, and brain perfusion may also be decreased. Insufficient energy production for demand can lead to intracellular sodium accumulation, calcium influx and cell death. Magnetic resonance (MR) is a promising technique to investigate these pathology driven hypotheses in vivo. MR spectroscopy can inform on mitochondrial function with measures of N acetyl aspartate (NAA), and requirement for extra-mitochondrial glycolysis via measurement of lactate. MR measurement of phosphorous (31P) and sodium (23Na) allows direct assessment of energy availability and axonal sodium handling. MR techniques for imaging perfusion can quantify oxygen delivery and nascent MR techniques that exploit the paramagnetism of deoxyhaemaglobin may be able to quantify oxygen utilization. This report reviews the physical principles underlying these techniques, their implementation for human in vivo imaging, and their application in neurological conditions with an emphasis on multiple sclerosis. Combination of these techniques to obtain a comprehensive picture of oxygen delivery, energy production and utilization may provide new insights into the pathophysiology of multiple sclerosis and may provide outcome measures for trials of novel treatments.





Similar content being viewed by others
References
Compston A, Confavreux C, McDonald I, Miller DH, Noseworthy J, Smith KJ et al (2006) McAlpine’s multiple sclerosis, 4th edn. Churchill Livingstone, London
Kremenchutzky M, Rice G, Baskerville J, Wingerchuk D, Ebers G (2006) The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain 129:584–594
Scalfari A, Neuhaus A, Degenhardt A, Rice GP, Muraro PA, Daumer M et al (2011) The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain 133:1914–1929
Metz I, Lucchinetti CF, Openshaw H, Garcia-Merino A, Lassmann H, Freedman MS et al (2007) Autologous haematopoietic stem cell transplantation fails to stop demyelination and neurodegeneration in multiple sclerosis. Brain 130:1254–1262
Kapoor R (2006) Neuroprotection in multiple sclerosis: therapeutic strategies and clinical trial design. Curr Opin Neurol 19:255–259
Dutta R, Trapp BD (2007) Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology 68:S22–S31
Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L (1998) Axonal transection in the lesions of Multiple Sclerosis. N Engl J Med 338:278–285
Kornek B, Storch MK, Weissert R, Wallstroem E, Stefferl A, Olsson T et al (2000) Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol 157:267–276
Craner M, Newcombe J, Black J, Hartle C, Cuzner M, Waxman S (2004) Molecular changes in neurons in multiple sclerosis: altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2+ exchanger. Proc Natl Acad Sci USA 101:8168–8173
Felts PA, Baker TA, Smith KJ (1997) Conduction in segmentally demyelinated mammalian central axons. J Neurosci 17:7267–7277
Ames A (2000) CNS energy metabolism as related to function. Brain Res Brain Res Rev 34:42–68
Witte ME, Bø L, Rodenburg RJ, Belien JA, Musters R, Hazes T et al (2009) Enhanced number and activity of mitochondria in multiple sclerosis lesions. J Pathol 219:193–204
Blokhin A, Vyshkina T, Komoly S, Kalman B (2008) Variations in mitochondrial DNA copy numbers in MS brains. J Mol Neurosci 35:283–287
Mahad DJ, Ziabreva I, Campbell G, Lax N, White K, Hanson PS et al (2009) Mitochondrial changes within axons in multiple sclerosis. Brain 132:1161–1174
Lu F, Selak M, O’Connor J, Croul S, Lorenzana C, Butunoi C et al (2000) Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J Neurol Sci 177:95–103
Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T et al (2006) Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol 59:478–489
Mahad D, Ziabreva I, Lassmann H, Turnbull D (2008) Mitochondrial defects in acute multiple sclerosis lesions. Brain 131:1722–1735
Gray E, Thomas TL, Betmouni S, Scolding N, Love S (2008) Elevated activity and microglial expression of myeloperoxidase in demyelinated cerebral cortex in multiple sclerosis. Brain Pathol 18:86–95
Rejdak K, Eikelenboom MJ, Petzold A, Thompson EJ, Stelmasiak Z, Lazeron RHC et al (2004) CSF nitric oxide metabolites are associated with activity and progression of multiple sclerosis. Neurology 63:1439–1445
Smith KJ, Lassmann H (2002) The role of nitric oxide in multiple sclerosis. Lancet Neurol. 1:232–241
Garthwaite G, Goodwin DA, Batchelor AM, Leeming K, Garthwaite J (2002) Nitric oxide toxicity in CNS white matter: an in vitro study using rat optic nerve. Neuroscience 109:145–155
Smith KJ, Kapoor R, Felts PA (1999) Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol 9:69–92
Lycke J, Wikkelsö C, Bergh AC, Jacobsson L, Andersen O (1993) Regional cerebral blood flow in multiple sclerosis measured by single photon emission tomography with technetium-99 m hexamethylpropyleneamine oxime. Eur Neurol 33:163–167
Brooks DJ, Leenders KL, Head G, Marshall J, Legg NJ, Jones T (1984) Studies on regional cerebral oxygen utilisation and cognitive function in multiple sclerosis. J Neurol Neurosurg Psychiatr 47:1182–1191
Sun X, Tanaka M, Kondo S, Okamoto K, Hirai S (1998) Clinical significance of reduced cerebral metabolism in multiple sclerosis: a combined PET and MRI study. Ann Nucl Med 12:89–94
Saindane AM, Law M, Ge Y, Johnson G, Babb JS, Grossman RI (2007) Correlation of diffusion tensor and dynamic perfusion MR imaging metrics in normal-appearing corpus callosum: support for primary hypoperfusion in multiple sclerosis. Am J Neuroradiol 28:767–772
Lazzarino G, Amorini A, Eikelenboom M, Killestein J, Belli A, Di Pietro V et al (2010) Cerebrospinal fluid ATP metabolites in multiple sclerosis. Mult Scler 16:549–554
Kapoor R, Davies M, Blaker PA, Hall SM, Smith KJ (2003) Blockers of sodium and calcium entry protect axons from nitric oxide-mediated degeneration. Ann Neurol 53:174–180
Petzold A, Gveric D, Groves M, Schmierer K, Grant D, Chapman M et al (2008) Phosphorylation and compactness of neurofilaments in multiple sclerosis: indicators of axonal pathology. Exp Neurol 213:326–335
Katz D, Taubenberger JK, Cannella B, McFarlin DE, Raine CS, McFarland HF (1993) Correlation between magnetic resonance imaging findings and lesion development in chronic, active multiple sclerosis. Ann Neurol 34:661–669
Miller DH, Barkhof F, Frank JA, Parker GJM, Thompson AJ (2002) Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain 125:1676–1695
Chen JT, Collins DL, Atkins HL, Freedman MS, Arnold DL (2008) Magnetization transfer ratio evolution with demyelination and remyelination in multiple sclerosis lesions. Ann Neurol 63:254–262
Frank JA (2004) Interferon-β-1b slows progression of atrophy in RRMS: three-year follow-up in NAb- and NAb patients. Neurology 62:719–725
Kapoor R, Furby J, Hayton T, Smith KJ, Altmann DR, Brenner R et al (2010) Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol 9:681–688
Miller DH, Soon D, Fernando KT, MacManus DG, Barker GJ, Yousry TA et al (2007) MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology 68:1390–1401
Zivadinov R, Reder AT, Filippi M, Minagar A, Stüve O, Lassmann H et al (2008) Mechanisms of action of disease-modifying agents and brain volume changes in multiple sclerosis. Neurology 71:136–144
Kirov II, Patil V, Babb JS, Rusinek H, Herbert J, Gonen O (2009) MR spectroscopy indicates diffuse multiple sclerosis activity during remission. J Neurol Neurosurg Psychiatr 80(12):1330–1336
Bagory M, Durand-Dubief F, Ibarrola D, Confavreux C, Sappey-Marinier D (2007) “Absolute” quantification in Magnetic Resonance Spectroscopy: validation of a clinical protocol in multiple sclerosis. Conf Proc IEEE Eng Med Biol Soc 2007:3458–3461
Provencher SW (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30:672–679
Pan JW, Takahashi K (2005) Interdependence of N-acetyl aspartate and high-energy phosphates in healthy human brain. Ann Neurol 57:92–97
Patel T, Clark J (1979) Synthesis of N-acetyl-l-aspartate by rat brain mitochondria and its involvement in mitochondrial/cytosolic carbon transport. Biochem J 184:539–546
Demougeot C, Garnier P, Mossiat C, Bertrand N, Giroud M, Beley A et al (2001) N-Acetylaspartate, a marker of both cellular dysfunction and neuronal loss: Its relevance to studies of acute brain injury. J Neurochem 77:408–415
Namboodiri AMA, Peethambaran A, Mathew R, Sambhu PA, Hershfield J, Moffett JR et al (2006) Canavan disease and the role of N-acetylaspartate in myelin synthesis. Mol Cell Endocrinol 252:216–223
Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AMA (2007) N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol 81:89–131
Ito H, Mori K, Harada M, Minato M, Naito E, Takeuchi M et al (2008) Serial brain imaging analysis of stroke-like episodes in MELAS. Brain Dev 30:483–488
Aragão MDFV, Law M, Netto JP, Valença MM, Naidich T (2009) Prognostic value of proton magnetic resonance spectroscopy findings in near drowning patients: reversibility of the early metabolite abnormalities relates with a good outcome. Arq Neuropsiquiatr 67:55–57
Parsons MW, Li T, Barber PA, Yang Q, Darby DG, Desmond PM et al (2000) Combined 1H MR spectroscopy and diffusion-weighted MRI improves the prediction of stroke outcome. Neurology 55:498–506
Cvoro V, Marshall I, Armitage PA, Bastin ME, Carpenter T, Rivers CS et al (2010) MR diffusion and perfusion parameters: relationship to metabolites in acute ischaemic stroke. J Neurol Neurosurg Psychiatr 81:185–191
Doelken MT, Stefan H, Pauli E, Stadlbauer A, Struffert T, Engelhorn T et al (2008) (1)H-MRS profile in MRI positive- versus MRI negative patients with temporal lobe epilepsy. Seizure 17:490–497
Li LM, Cendes F, Bastos AC, Andermann F, Dubeau F, Arnold DL (1998) Neuronal metabolic dysfunction in patients with cortical developmental malformations: a proton magnetic resonance spectroscopic imaging study. Neurology 50:755–759
Cendes F, Andermann F, Dubeau F, Matthews PM, Arnold DL (1997) Normalization of neuronal metabolic dysfunction after surgery for temporal lobe epilepsy: evidence from proton MR spectroscopic imaging. Neurology 49:1525–1533
Wang Z, Zhao C, Yu L, Zhou W, Li K (2009) Regional metabolic changes in the hippocampus and posterior cingulate area detected with 3 Tesla magnetic resonance spectroscopy in patients with mild cognitive impairment and Alzheimer disease. Acta Radiol 50:312–319
Mielke R, Schopphoff HH, Kugel H, Pietrzyk U, Heindel W, Kessler J et al (2001) Relation between 1H MR spectroscopic imaging and regional cerebral glucose metabolism in Alzheimer’s disease. Int J Neurosci 107:233–245
Munoz Maniega S, Cvoro V, Chappell FM, Armitage PA, Marshall I, Bastin ME et al (2008) Changes in NAA and lactate following ischemic stroke: a serial MR spectroscopic imaging study. Neurology 71:1993–1999
Kreis R, Arcinue E, Ernst T, Shonk TK, Flores R, Ross BD (1996) Hypoxic encephalopathy after near-drowning studied by quantitative 1H-magnetic resonance spectroscopy. J Clin Invest 97:1142–1154
Sajja BR, Wolinsky JS, Narayana PA (2009) Proton magnetic resonance spectroscopy in multiple sclerosis. Neuroimaging Clin N Am 19:45–58
Caramanos Z, Narayanan S, Arnold DL (2005) 1H-MRS quantification of tNA and tCr in patients with multiple sclerosis: a meta-analytic review. Brain 128:2483–2506
Bitsch A, Bruhn H, Vougioukas V, Stringaris A, Lassmann H, Frahm J et al (1999) Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR 20:1619–1627
De Stefano N, Iannucci G, Sormani MP, Guidi L, Bartolozzi ML, Comi G et al (2002) MR correlates of cerebral atrophy in patients with multiple sclerosis. J Neurol 249:1072–1077
Wylezinska M, Cifelli A, Jezzard P, Palace J, Alecci M, Matthews PM (2003) Thalamic neurodegeneration in relapsing-remitting multiple sclerosis. Neurology 60:1949–1954
Ranjeva JP, Pelletier J, Confort-Gouny S, Ibarrola D, Audoin B, Le Fur Y et al (2003) MRI/MRS of corpus callosum in patients with clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler 9:554–565
Cader S, Johansen-Berg H, Wylezinska M, Palace J, Behrens TE, Smith S et al (2007) Discordant white matter N-acetylaspartate and diffusion MRI measures suggest that chronic metabolic dysfunction contributes to axonal pathology in multiple sclerosis. Neuroimage 36:19–27
Audoin B, Ibarrola D, Malikova I, Soulier E, Confort-Gouny S, Duong MVA et al (2007) Onset and underpinnings of white matter atrophy at the very early stage of multiple sclerosis–a two-year longitudinal MRI/MRSI study of corpus callosum. Mult Scler 13:41–51
Mathiesen HK, Jonsson A, Tscherning T, Hanson LG, Andresen J, Blinkenberg M et al (2006) Correlation of global N-acetyl aspartate with cognitive impairment in multiple sclerosis. Arch Neurol 63:533–536
Davie CA, Hawkins CP, Barker GJ, Brennan A, Tofts PS, Miller DH et al (1994) Serial proton magnetic resonance spectroscopy in acute multiple sclerosis lesions. Brain 117:49–58
De Stefano N, Matthews PM, Antel JP, Preul M, Francis G, Arnold DL (1995) Chemical pathology of acute demyelinating lesions and its correlation with disability. Ann Neurol 38:901–909
Narayana PA, Doyle TJ, Lai D, Wolinsky JS (1998) Serial proton magnetic resonance spectroscopic imaging, contrast-enhanced magnetic resonance imaging, and quantitative lesion volumetry in multiple sclerosis. Ann Neurol 43:56–71
Zaaraoui W, Rico A, Audoin B, Reuter F, Malikova I, Soulier E et al (2010) Unfolding the long-term pathophysiological processes following an acute inflammatory demyelinating lesion of multiple sclerosis. Magn Reson Imaging 28:477–486
De Stefano N, Narayanan S, Matthews PM, Francis GS, Antel JP, Arnold DL (1999) In vivo evidence for axonal dysfunction remote from focal cerebral demyelination of the type seen in multiple sclerosis. Brain 122:1933–1939
Tiberio M, Chard DT, Altmann DR, Davies G, Griffin CM, McLean MA et al (2006) Metabolite changes in early relapsing-remitting multiple sclerosis. a two year follow-up study. J Neurol 253:224–230
Tourbah A, Stievenart JL, Gout O, Fontaine B, Liblau R, Lubetzki C et al (1999) Localized proton magnetic resonance spectroscopy in relapsing remitting versus secondary progressive multiple sclerosis. Neurology 53:1091–1097
Sarchielli P, Presciutti O, Pelliccioli GP, Tarducci R, Gobbi G, Chiarini P et al (1999) Absolute quantification of brain metabolites by proton magnetic resonance spectroscopy in normal-appearing white matter of multiple sclerosis patients. Brain 122:513–521
Ruiz-Peña JL, Piñero P, Sellers G, Argente J, Casado A, Foronda J et al (2004) Magnetic resonance spectroscopy of normal appearing white matter in early relapsing-remitting multiple sclerosis: correlations between disability and spectroscopy. BMC Neurol 4:8
Fu L, Matthews PM, De Stefano N, Worsley KJ, Narayanan S, Francis GS et al (1998) Imaging axonal damage of normal-appearing white matter in multiple sclerosis. Brain 121:103–113
De Stefano N, Matthews PM, Fu L, Narayanan S, Stanley J, Francis GS et al (1998) Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain 121:1469–1477
Davie CA, Barker GJ, Webb S, Tofts PS, Thompson AJ, Harding AE et al (1995) Persistent functional deficit in multiple sclerosis and autosomal dominant cerebellar ataxia is associated with axon loss. Brain 118:1583–1592
Bjartmar C, Kidd G, Mörk S, Rudick R, Trapp BD (2000) Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol 48:893–901
Magistretti PJ, Pellerin L, Rothman DL, Shulman RG (1999) Energy on demand. Science 283:496–497
Bergersen LH (2007) Is lactate food for neurons? Comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience 145:11–19
Gallagher CN, Carpenter KLH, Grice P, Howe DJ, Mason A, Timofeev I et al (2009) The human brain utilizes lactate via the tricarboxylic acid cycle: a 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain 132:2839–2849
Magistretti PJ, Pellerin L (1999) Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci 354:1155–1163
Soares D, Law M (2009) Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clin Radiol 64:12–21
Tzoulis C, Neckelmann G, Mork SJ, Engelsen BE, Viscomi C, Moen G et al (2010) Localized cerebral energy failure in DNA polymerase gamma-associated encephalopathy syndromes. Brain 133:1428–1437
Saneto RP, Friedman SD, Shaw DWW (2008) Neuroimaging of mitochondrial disease. Mitochondrion 8:396–413
Henchcliffe C, Shungu DC, Mao X, Huang C, Nirenberg MJ, Jenkins BG et al (2008) Multinuclear magnetic resonance spectroscopy for in vivo assessment of mitochondrial dysfunction in Parkinson’s disease. Ann NY Acad Sci 1147:206–220
Li X, Vigneron DB, Cha S, Graves EE, Crawford F, Chang SM et al (2005) Relationship of MR-derived lactate, mobile lipids, and relative blood volume for gliomas in vivo. AJNR 26:760–769
Hillary FG, Liu WC, Genova HM, Maniker AH, Kepler K, Greenwald BD et al (2007) Examining lactate in severe TBI using proton magnetic resonance spectroscopy. Brain Inj 21:981–991
Canas N, Soares P, Calado S, Pestana R, Ribeiro C, Vale J (2010) Pathophysiology and long-term outcome of reversible tumor-like lesions induced by presenting status epilepticus. J Neuroimaging 20:169–174
Chen CJ (2001) Serial proton magnetic resonance spectroscopy in lesions of Balò concentric sclerosis. J Comput Assist Tomogr 25:713–718
Law M, Meltzer DE, Cha S (2002) Spectroscopic magnetic resonance imaging of a tumefactive demyelinating lesion. Neuroradiology. 44:986–989
Simone IL, Federico F, Trojano M, Tortorella C, Liguori M, Giannini P et al (1996) High resolution proton MR spectroscopy of cerebrospinal fluid in MS patients. Comparison with biochemical changes in demyelinating plaques. J Neurol Sci 144:182–190
Schocke MFH, Berger T, Felber SR, Wolf C, Deisenhammer F, Kremser C et al (2003) Serial contrast-enhanced magnetic resonance imaging and spectroscopic imaging of acute multiple sclerosis lesions under high-dose methylprednisolone therapy. Neuroimage 20:1253–1263
Barbiroli B, Montagna P, Martinelli P, Lodi R, Iotti S, Cortelli P et al (1993) Defective brain energy metabolism shown by in vivo 31P MR spectroscopy in 28 patients with mitochondrial cytopathies. J Cereb Blood Flow Metab 13:469–474
Yoshida K, Furuse M, Izawa A, Iizima N, Kuchiwaki H, Inao S (1996) Dynamics of cerebral blood flow and metabolism in patients with cranioplasty as evaluated by 133Xe CT and 31P magnetic resonance spectroscopy. J Neurol Neurosurg Psychiatr 61:166–171
Chu WJ, Hetherington HP, Kuzniecky RI, Simor T, Mason GF, Elgavish GA (1998) Lateralization of human temporal lobe epilepsy by 31P NMR spectroscopic imaging at 4.1 T. Neurology 51:472–479
Levine SR, Helpern JA, Welch KM, Vande Linde AM, Sawaya KL, Brown EE et al (1992) Human focal cerebral ischemia: evaluation of brain pH and energy metabolism with P-31 NMR spectroscopy. Radiology 185:537–544
Stamelou M, Pilatus U, Reuss A, Magerkurth J, Eggert KM, Knake S et al (2009) In vivo evidence for cerebral depletion in high-energy phosphates in progressive supranuclear palsy. J Cereb Blood Flow Metab 29:861–870
Hu MTM, Brooks DJ, Taylor-Robinson SD, Chaudhuri KR, Bell JD, Labbé C et al (2000) Cortical dysfunction in non-demented Parkinson’s disease patients. Brain 123:340–352
Cadoux-Hudson TA, Kermode A, Rajagopalan B, Taylor D, Thompson AJ, Ormerod IE et al (1991) Biochemical changes within a multiple sclerosis plaque in vivo. J Neurol Neurosurg Psychiatr 54:1004–1006
Husted CA, Goodin DS, Hugg JW, Maudsley AA, Tsuruda JS, de Bie SH et al (1994) Biochemical alterations in multiple sclerosis lesions and normal-appearing white matter detected by in vivo 31P and 1H spectroscopic imaging. Ann Neurol 36:157–165
Ouwerkerk R (2007) Sodium magnetic resonance imaging: from research to clinical use. J Am Coll Radiol 4:739–741
Maudsley AA, Hilal SK (1984) Biological aspects of sodium-23 imaging. Br Med Bull 40:165–166
Ouwerkerk R, Bleich KB, Gillen JS, Pomper MG, Bottomley PA (2003) Tissue sodium concentration in human brain tumors as measured with 23Na MR imaging. Radiology 227:529–537
Ouwerkerk R, Jacobs MA, Macura KJ, Wolff AC, Stearns V, Mezban SD et al (2007) Elevated tissue sodium concentration in malignant breast lesions detected with non-invasive 23Na MRI. Breast Cancer Res Treat 106:151–160
Hussain MS, Stobbe RW, Bhagat YA, Emery D, Butcher KS, Manawadu D et al (2009) Sodium imaging intensity increases with time after human ischemic stroke. Ann Neurol 66:55–62
Inglese M, Madelin G, Oesingmann N, Babb JS, Wu W, Stoeckel B et al (2010) Brain tissue sodium concentration in multiple sclerosis: a sodium imaging study at 3 Tesla. Brain 133:847–857
Hancu I, Boada FE, Shen GX (1999) Three-dimensional triple-quantum-filtered 23Na imaging of in vivo human brain. Magn Reson Med 42:1146–1154
LaVerde G, Nemoto E, Jungreis CA, Tanase C, Boada FE (2007) Serial triple quantum sodium MRI during non-human primate focal brain ischemia. Magn Reson Med 57(1):201–205
Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR (1996) High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med 36:715–725
Parsons MW, Yang Q, Barber PA, Darby DG, Desmond PM, Gerraty RP et al (2001) Perfusion Magnetic Resonance Imaging maps in hyperacute stroke : relative cerebral blood flow most accurately identifies tissue destined to infarct. Stroke 32:1581–1587
Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E et al (2006) Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 60(5):508–517
Grandin CB, Duprez TP, Smith AM, Oppenheim C, Peeters A, Robert AR et al (2002) Which MR-derived perfusion parameters are the best predictors of infarct growth in hyperacute stroke? Comparative study between relative and quantitative measurements. Radiology 223(2):361–370
Toth G, Albers GW (2000) Use of MRI to estimate the therapeutic window in acute stroke: is perfusion-weighted imaging/diffusion-weighted imaging mismatch an EPITHET for salvageable ischemic brain tissue? Stroke 40:333–335
Hakyemez B, Erdogan C, Ercan I, Ergin N, Uysal S, Atahan S (2005) High-grade and low-grade gliomas: differentiation by using perfusion MR imaging. Clin Radiol 60:493–502
Akella NS, Twieg DB, Mikkelsen T, Hochberg FH, Grossman S, Cloud GA et al (2004) Assessment of brain tumor angiogenesis inhibitors using perfusion magnetic resonance imaging: quality and analysis results of a phase I trial. J Magn Reson Imaging 20:913–922
Cha S, Knopp EA, Johnson G, Wetzel SG, Litt AW, Zagzag D (2002) Intracranial mass lesions: dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology 223:11–29
Haselhorst R, Kappos L, Bilecen D, Scheffler K, Möri D, Radü EW et al (2000) Dynamic susceptibility contrast MR imaging of plaque development in multiple sclerosis: application of an extended blood-brain barrier leakage correction. J Magn Reson Imaging 11:495–505
Ge Y, Law M, Johnson G, Herbert J, Babb JS, Mannon LJ et al (2005) Dynamic susceptibility contrast perfusion MR imaging of multiple sclerosis lesions: characterizing hemodynamic impairment and inflammatory activity. AJNR 26:1539–1547
Varga AW, Johnson G, Babb JS, Herbert J, Grossman RI, Inglese M (2009) White matter hemodynamic abnormalities precede sub-cortical gray matter changes in multiple sclerosis. J Neurol Sci 282:28–33
Law M, Saindane AM, Ge Y, Babb JS, Johnson G, Mannon LJ et al (2004) Microvascular abnormality in relapsing-remitting multiple sclerosis: perfusion MR imaging findings in normal-appearing white matter. Radiology 231:645–652
Adhya S, Johnson G, Herbert J, Jaggi H, Babb JS, Grossman RI et al (2006) Pattern of hemodynamic impairment in multiple sclerosis: dynamic susceptibility contrast perfusion MR imaging at 3.0 T. Neuroimage 33:1029–1035
Inglese M, Adhya S, Johnson G, Babb JS, Miles L, Jaggi H et al (2008) Perfusion magnetic resonance imaging correlates of neuropsychological impairment in multiple sclerosis. J Cereb Blood Flow Metab 28:164–171
Inglese M, Park S, Johnson G, Babb JS, Miles L, Jaggi H et al (2007) Deep gray matter perfusion in multiple sclerosis: dynamic susceptibility contrast perfusion magnetic resonance imaging at 3 T. Arch Neurol 64:196–202
Hasebroock KM, Serkova NJ (2009) Toxicity of MRI and CT contrast agents. Expert Opin Drug Metab Toxicol 5:403–416
Paiva FF, Tannús A, Silva AC (2007) Measurement of cerebral perfusion territories using arterial spin labelling. NMR Biomed 20:633–642
Petersen ET, Zimine I, Ho YL, Golay X (2006) Non-invasive measurement of perfusion: a critical review of arterial spin labelling techniques. Br J Radiol 79:688–701
Ye FQ, Mattay VS, Jezzard P, Frank JA, Weinberger DR, McLaughlin AC (1997) Correction for vascular artifacts in cerebral blood flow values measured by using arterial spin tagging techniques. Magn Reson Med 37:226–235
van Osch MJP, Teeuwisse WM, van Walderveen MAA, Hendrikse J, Kies DA, van Buchem MA (2009) Can arterial spin labelling detect white matter perfusion signal? Magn Reson Med 62:165–173
Yoshiura T, Hiwatashi A, Noguchi T, Yamashita K, Ohyagi Y, Monji A et al (2009) Arterial spin labelling at 3-T MR imaging for detection of individuals with Alzheimer’s disease. Eur Radiol 19(12):2819–2825
Rule RR, Schuff N, Miller RG, Weiner MW (2010) Gray matter perfusion correlates with disease severity in ALS. Neurology 74:821–827
Ge Y, Patel MB, Chen Q, Grossman EJ, Zhang K, Miles L et al (2009) Assessment of thalamic perfusion in patients with mild traumatic brain injury by true FISP arterial spin labelling MR imaging at 3T. Brain Inj 23:666–674
Horn H, Federspiel A, Wirth M, Müller TJ, Wiest R, Wang J et al (2009) Structural and metabolic changes in language areas linked to formal thought disorder. Br J Psychiatry 194:130–138
Rashid W, Parkes LM, Ingle GT, Chard DT, Toosy AT, Altmann DR et al (2004) Abnormalities of cerebral perfusion in multiple sclerosis. J Neurol Neurosurg Psychiatr 75:1288–1293
De Keyser J, Steen C, Mostert JP, Koch MW (2008) Hypoperfusion of the cerebral white matter in multiple sclerosis: possible mechanisms and pathophysiological significance. J Cereb Blood Flow Metab 28:1645–1651
Ogawa S, Lee TM, Nayak AS, Glynn P (1990) Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med 14:68–78
Silvennoinen MJ, Clingman CS, Golay X, Kauppinen RA, van Zijl PCM (2003) Comparison of the dependence of blood R2 and R2* on oxygen saturation at 1.5 and 4.7 Tesla. Magn Reson Med 49:47–60
Yablonskiy DA, Haacke EM (1994) Theory of NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn Reson Med 32:749–763
He X, Yablonskiy DA (2007) Quantitative BOLD: mapping of human cerebral deoxygenated blood volume and oxygen extraction fraction: default state. Magn Reson Med 57:115–126
He X, Zhu M, Yablonskiy DA (2008) Validation of oxygen extraction fraction measurement by qBOLD technique. Magn Reson Med 60:882–888
Xu F, Ge Y, Lu H (2009) Noninvasive quantification of whole-brain cerebral metabolic rate of oxygen (CMRO2) by MRI. Magn Reson Med 62:141–148
Haacke EM, Cheng NYC, House MJ, Liu Q, Neelavalli J, Ogg RJ et al (2005) Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 23:1–25
Holst B, Siemonsen S, Finsterbusch J, Bester M, Schippling S, Martin R et al (2009) T2′ imaging indicates decreased tissue metabolism in frontal white matter of MS patients. Mult Scler 15:701–707
An H, Lin W (2002) Cerebral oxygen extraction fraction and cerebral venous blood volume measurements using MRI: effects of magnetic field variation. Magn Reson Med 47:958–966
Golay X, Silvennoinen MJ, Zhou J, Clingman CS, Kauppinen RA, Pekar JJ et al (2001) Measurement of tissue oxygen extraction ratios from venous blood T(2): increased precision and validation of principle. Magn Reson Med 46:282–291
Lu H, Ge Y (2008) Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn Reson Med 60:357–363
Barnes SRS, Haacke EM (2009) Susceptibility weighted imaging: clinical angiographic applications. Magn Reson Imaging Clin N Am 17:47–61
Ge Y, Zohrabian VM, Osa E, Xu J, Jaggi H, Herbert J et al (2009) Diminished visibility of cerebral venous vasculature in multiple sclerosis by susceptibility-weighted imaging at 3.0 Tesla. J Magn Reson Imaging 29:1190–1194
Trapp BD, Stys PK (2009) Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol 8:280–291
Acknowledgments
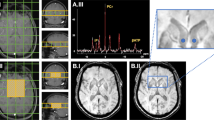
The NMR Research Unit is supported by the UK Multiple Sclerosis Society and University College London and University College London Hospitals Comprehensive Biomedical Research Centre. The MR images illustrated in the review were obtained on a Philips 3T Achieva scanner.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paling, D., Golay, X., Wheeler-Kingshott, C. et al. Energy failure in multiple sclerosis and its investigation using MR techniques. J Neurol 258, 2113–2127 (2011). https://doi.org/10.1007/s00415-011-6117-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-011-6117-7