Abstract
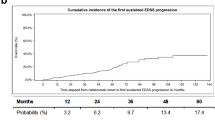
Natalizumab is the first selective adhesion molecule inhibitor indicated for treatment of active relapsing-remitting multiple sclerosis (RRMS). Natalizumab has been available in France since April 2007. The aims of this study are to analyze demographic, clinical, and tolerance data from French patients with RRMS treated with natalizumab in actual clinical practice and to draw comparisons with patients in the pivotal AFFIRM study. All patients with RRMS in the Nord-Pas de Calais and Alsace regions of France treated with natalizumab at any time since April 2007 were included. Variables analyzed included previous treatments; disability status [Expanded Disability Status Scale (EDSS) score]; annualized relapse rate (ARR) at baseline and after 12 months of treatment; and adverse events. Data from 384 patients (72% female) were evaluated. Mean baseline EDSS score was 3.53 and mean baseline ARR was 2.19, both significantly greater than in AFFIRM. One hundred twenty-seven patients completed 12 months of treatment; mean EDSS score in this group was 3.02 (14% reduction) and mean ARR was 0.59 (73% reduction). Although these patients had significantly different baseline characteristics and greater disability compared with patients receiving natalizumab in AFFIRM, average disability remained stable and ARR declined by 73%. Tolerability was similar to that observed in AFFIRM.




Similar content being viewed by others
References
Agence Française de Sécurité Sanitaire des Produits de Santé. Bon usage; Mise au point: Utilisation de la spécialité TYSABRI® 300 mg (natalizumab) dans le traitement de la sclérose en plaques [PDF in French]. http://www.afssaps.fr/content/download/6205/60160/version/4/file/mise_au_tysabri.pdf. Accessed 22 Mar 2009
Biogen Idec, Inc. TYSABRI update. http://www.biogenidec.com/site/tysabri-information-center.html. Accessed 26 Mar 2009
Calabresi PA, Giovannoni G, Confavreux C, Galetta SL, Havrdova E, Hutchinson M, Kappos L, Miller DH, O’Connor PW, Phillips JT, Polman CH, Radue EW, Rudick RA, Stuart WH, Lublin FD, Wajgt A, Weinstock-Guttman B, Wynn DR, Lynn F, Panzara MA (2007) The incidence and significance of anti-natalizumab antibodies: results from AFFIRM and SENTINEL. Neurology 69:1391–1403
Confavreux C, Vukusic S (2006) Natural history of multiple sclerosis: a unifying concept. Brain 129:606–616
European Medicines Agency. TYSABRI European public assessment report: scientific discussion. http://www.emea.europa.eu/humandocs/PDFs/EPAR/tysabri/H-603-en6.pdf. Accessed 31 Mar 2009
Hartung HP (2009) New cases of progressive multifocal leukoencephalopathy after treatment with natalizumab. Lancet Neurol 8:28–30
Lublin FD, Reingold SC (1996) Defining the clinical course of multiple sclerosis: results of an international survey. Neurology 46:907–911
Mancardi GL, Amato MP, D’Alessandro R, Drago F, Milanese C, Popoli P, Provinciali L, Rossi P, Savettieri G, Tedeschi G, Rosaria Tola M, Vanacore N, Covezzoli A, De Rosa M, Piccinni C, Montarano N, Periotto L, Addis A, Martini N (2008) Natalizumab: a country-based surveillance program. Neurol Sci 29(Suppl 2):S235–S237
Munschauer F, Giovannoni G, Lublin F, O’Connor PW, Phillips JT, Polman CH, Pace A, Kim R, Panzara MA (2008) Natalizumab significantly increases the cumulative probability of sustained improvement in physical disability. Poster 474 ACTRIMS/ECTRIMS 2008
Oturai AB, Koch-Henriksen N, Petersen T, Jensen PEH, Sellebjerg F, Sorensen PS (2009) Efficacy of natalizumab in multiple sclerosis patients with high disease activity: a Danish nationwide study. Eur J Neurol 16:420–423
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips T, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354:899–910
Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O’Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald criteria”. Ann Neurol 58:840–846
Putzki N, Kollila K, Woods S, Igwe E, Diener HC, Limmroth V (2009) Natalizumab is effective as second line therapy in the treatment of relapsing remitting multiple sclerosis. Eur J Neurol 16:424–426
Schumacher GA, Beebe G, Kibler RF, Kurland LT, Kurtzke JF, McDowell F (1965) Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Ann NY Acad Sci 122:552–568
Acknowledgments
The authors thank the neurologists who participated in the ALSACEP and GSEP networks and who contributed to the follow-up of this cohort of patients: A. Bahn, S. Dufour-Delalande, D. Ferriby (CH. Tourcoing); G. Calais, C. Crinquette, P. Hautecoeur, A. Manceaux (CH. Saint Philibert); S. Courtois, F. Derouiche, E. Muller (CH. Mulhouse); P. Devos (CH. Boulogne sur Mer); AC. Lepoutre, M. Marcel (CH. Lens); A. Monjour, C. Renglewicz-Destuynde, F. Sellal (CH. Colmar); and A. Verier (CH Valenciennes). Michael Theisen, Ann Marie Galioto, and Matthew Hasson, Scientific Connexions, Newtown, PA, USA provided technical and editorial support during the preparation of this manuscript for submission; funding for these activities was provided by Biogen Idec, Inc. and Elan Pharmaceuticals, Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the Groupe Septentrional d’Etudes et de Recherche sur la Sclérose en Plaques (GSEP) and ALSACEP Networks.
Rights and permissions
About this article
Cite this article
Outteryck, O., Ongagna, JC., Zéphir, H. et al. Demographic and clinic characteristics of French patients treated with natalizumab in clinical practice. J Neurol 257, 207–211 (2010). https://doi.org/10.1007/s00415-009-5294-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-009-5294-0