Abstract
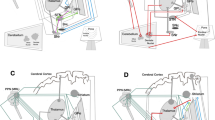
In this review, we have tried to elucidate substrates for the execution of normal gait and to understand pathophysiological mechanisms of gait failure in basal ganglia dysfunctions. In Parkinson’s disease, volitional and emotional expressions of movement processes are seriously affected in addition to the disturbance of automatic movement processes, such as adjustment of postural muscle tone before gait initiation and rhythmic limb movements during walking. These patients also suffer from muscle tone rigidity and postural instability, which may also cause reduced walking capabilities in adapting to various environments. Neurophysiological and clinical studies have suggested the importance of basal ganglia connections with the cerebral cortex and limbic system in the expression of volitional and emotional behaviors. Here we hypothesize a crucial role played by the basal ganglia-brainstem system in the integrative control of muscle tone and locomotion. The hypothetical model may provide a rational explanation for the role of the basal ganglia in the control of volitional and automatic aspects of movements. Moreover, it might also be beneficial for understanding pathophysiological mechanisms of basal ganglia movement disorders. A part of this hypothesis has been supported by studies utilizing a constructive simulation engineering technique that clearly shows that an appropriate level of postural muscle tone and proper acquisition and utilization of sensory information are essential to maintain adaptable bodily functions for the full execution of bipedal gait.
In conclusion, we suggest that the major substrates for supporting bipedal posture and executing bipedal gait are 1) fine neural networks such as the cortico-basal ganglia loop and basal ganglia-brainstem system, 2) fine musculoskeletal structures with adequately developed (postural) muscle tone, and 3) proper sensory processing. It follows that any dysfunction of the above sensorimotor integration processes would result in gait disturbance.
Similar content being viewed by others
References
Alexander GE, Crutcher MD (1990) Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13:267–271
Armstrong DM (1986) Supraspinal contribution to the initiation and control of locomotion in the cat. Prog Neurobiol 26:273–361
Armstrong DM, Apps R, Marple-Horvat DE (1997) Aspects of cerebellar function in relation to locomotor movements. Prog Brain Res 114:401–420
Beckstead RM, Domesick VB, Nauta WJH (1979) Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res 175:191–217
Chase MH, Morales FR (1990) The atonia and myoclonia of active (REM) sleep. Ann Rev Psychol 41:557–584
Crowdy KA, Hollands MA, Ferguson IT, Marple-Horvat DE (2000) Evidence for interactive locomotor and oculomotor deficits in cerebellar patients during visually guided stepping. Exp Brain Res 135:437–454
Culebras A, Moore JT (1989) Magnetic resonance findings in REM sleep behavior disorder. Neurology 39:1519–1523
Delong MR (1990) Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13:281–289
Denny-Brown D (1962) The midbrain and motor integration. Proc R Soc Med 55:527–538
Drew T, Dubuc R, Rossignol S (1986) Discharge patterns of reticulospinal and other reticular neurons in chronic unrestrained cats walking on a treadmill. J Neurophysiol 55:375–401
Drew T, Jiang W, Kably B, Lavoie S (1996) Role of the motor cortex in the control of visually triggered gait modifications. Can J Physiol Pharmacol 7:426–442
Drew T, Prentice S, Schepens B (2004) Cortical and brainstem control of locomotion. Prog Brain Res 143:251–261
Georgopoulos AP, Grillner S (1989) Visuomotor coordination in reaching and locomotion. Science 245:1209–1210
Grillner S (1981) Control of locomotion in bipeds, tetrapods, and fish. In: Brooks VB (ed) The nervous system II. Am Physiol Soc Press, Bethesda, pp 1179–1236
Grillner S, Georgopoulos AP, Jordan LM (1997) Selection and initiation of motor behavior. In: Stein PSG, Grillner S, Selverson AI, Stuart DG (eds), Neurons, networks, and motor behavior, MIT Press, pp 3–19
Habaguchi T, Takakusaki K, Saitoh K, Sugimoto J, Sakamoto T (2002) Medullary reticulospinal tract mediating the generalized motor inhibition in cats: II. Functional organization within the medullary reticular formation with respect to postsynaptic inhibition of forelimb and hindlimb motoneurons. Neuroscience 113:65–77
Hanakawa T, Katsumi Y, Fukuyama H, Honda M, Hayashi T, Kimura J, Shibasaki H (1999) Mechanisms underlying gait disturbance in Parkinson’s disease: a single photon emission computed tomography study. Brain 122:1271–1282
Hikosaka O, Takikawa Y, Kawgoe R (2000) Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80:954–978
Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, Miyachi S, Doya K (1999) Parallel neural networks for learning sequential procedures. Trends Neurosci 22:464–471
Honda T, Semba K (1994) Serotonergic synaptic input to cholinergic neurons in the rat mesopontine tegmentum. Brain Res 647:299–306
Inglis WL, Winn P (1995) The pedunculopontine tegmental nucleus: where the striatum meets the reticular formation. Prog Neurobiol 47:1–29
Keizer K, Kuypers HGJM (1989) Distribution of corticospinal neurons with collaterals to the lower brain stem reticular formation in monkey (Macaca fascicularis). Exp Brain Res 74:311–318
Lajoie K, Drew T (2007) Lesions of area 5 of the posterior parietal cortex in the cat produce errors in the accuracy of paw placement during visually guided locomotion. J Neurophysiol 97:2339–2354
Leonald CS, Llinás R (1994) Serotonergic and cholinergic inhibition of mesopontine cholinergic neurons controlling REM sleep; an in vitro electrophysiological study. Neuroscience 59:309–330
Marple-Horvat DE, Criado JM, Armstrong DM (1998) Neuronal activity in the lateral cerebellum of the cat related to visual stimuli at rest, visually guided step modification, and saccadic eye movements. J Physiol 506:489–514
Marsden CD (1982) The mysterious motor function of the basal ganglia: The Robert Wartenberg Lecture. Neurology 32:514–539
Masdeu JC, Alampur U, Cavaliere R, Tavoulareas G (1994) Astasia and gait failure with damage of the pontomesencephalic locomotor region. Ann Neurol 35:619–621
Matsumura M, Nambu A, Yamaji Y, Watanabe K, Imai H, Inase M, Tokuno H, Takada M (2000) Organization of somatic motor inputs from the frontal lobe to the pedunculopontine tegmental nucleus in the macaque monkey. Neuroscience 98:97–110
Matsuyama K, Drew T (1997) Organization of the projections from the pericruciate cortex to the pontomedullary brainstem of the cat: a study using the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J Comp Neurol 389:617–641
McCrea DA, Rybak IA (2008) Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 57:134–146
McVea DA, Pearson KG (2007) Long-lasting, context-dependent modification of stepping in the cat after repeated stumbling-corrective responses. J Neurophysiol 97:659–669
Middleton FA, Strick PL (2000) Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Rev 31:236–250
Mileykovskiy BY, Kiyashchenko LI, Kodama T, Lai YY, Siegel JM (2000) Activation of pontine and medullary motor inhibitory regions reduces discharge in neurons located in the locus coeruleus and the anatomical equivalent of the midbrain locomotor region. J Neurosci 20:8551–8558
Mileykovskiy BY, Kiyashchenko LI, Siegel JM (2005) Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron 46:787–798
Mogenson GI (1991) The role of mesolimbic dopamine projections to the ventral striatum in response initiation. In: Shimamura M, Griller S, Edgerton VR (eds) Neurobiologicalbasis of human locomotion, Japan Scientific Press, Tokyo, pp 33–4
Mogensen GJ, Brudzynski SM, Wu M, Yang CR, Yim CCY (1993) From motivation to action: a review of dopaminergic regulation of limbic-nucleus accumbens-ventral pallidum-pedunculopontine nucleus circuits involved in limbic-motor integration. In: Kalivas PW (ed) Limbic motor circuit and neuropsychiatry. CRC Press, Boca Raton FL, pp 193–236
Mori F, Nakajima K, Tachibana A, Nambu A, Mori S (2003) Cortical mechanisms for the control of bipedal locomotion in Japanese monkeys: II. Local inactivation of the supplementary motor area (SMA). Neurosci Res 46(Suppl 1):S157
Mori S (1987) Integration of posture and locomotion in acute decerebrate cats and in awake, free moving cats. Prog Neurobiol 28:161–196
Mori S, Sakamoto T, Ohta Y, Takakusaki K, Matsuyama K (1989) Site-specific postural and locomotor changes evoked in awake, freely moving intact cats by stimulating the brainstem. Brain Res 505:66–74
Moriizumi T, Nakamura Y, Tokuno H, Kitao Y, Kudo M (1988) Topographic projections from the basal ganglia to the nucleus tegmenti pedunculopontinus pars compacta of the cat with special reference to pallidal projections. Exp Brain Res 71:298–306
Morris ME, Iansek R, Matyas TA, Summers JJ (1994) The pathogenesis of gait hypokinesia in Parkinson’s disease. Brain 117:1169–1181
Morton SM, Bastian AJ (2003) Relative contributions of balance and voluntary leg-coordination deficits to cerebellar gait ataxia. J Neurophysiol 89:1844–1856
Murray MP, Sepic SB, Gardner GM, Downs WJ (1978) Walking patterns of men with parkinsonism. Am J Phys Med 57:278–294
Nakajima K, Mori F, Tachibana A, Nambu A, Mori S (2003) Cortical mechanisms for the control of bipedal locomotion in Japanese monkeys: I. Local inactivation of the primary motor cortex (M1). Neurosci Res 46(Suppl 1):S156
Obeso JA, Rodriguez MC, DeLong MR (1997) Basal ganglia pathophysiology. In: Obeso JA, DeLong MR, Ohye C, Marsden CD (eds) The basal ganglia and new surgical approaches for Parkinson’s disease. Advance in Neurology, vol 74. Lippincott-Raven Publishers, Philadelphia, pp 3–18
Pahapill PA, Lozano AM (2000) The pedunculopontine nucleus and Parkinson’s disease. Brain 123:1767–1783
Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS (1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18:9996–10015
Rektor I, Rektorova I, Kubova D (2006) Vascular parkinsonism – an update. J Neurol Sci 248:185–191
Rossignol S (1996) Neural control of stereotypic limb movements. In: Rowell LB, Shepherd JT (eds) Handbook of physiology, section 12. Oxford University Press, New York, pp 173–216
Rossignol S, Dubuc R, Gossard J-P (2006) Dynamic sensorimotor interactions in locomotion. Physiol Rev 86:89–154
Saint-Cyr JA, Taylor AE, Nicholson K (1995) Behavior and the basal ganglia. In: Weiner WJ, Lang AE (eds) Behavioral neurology of movement disorders. Advance in Neurology, vol. 65. Raven Press, New York, pp 1–28
Sakurai T (2002) Roles of orexins in regulation of feeding and wakefulness. Neuroreport 13:987–995
Semba K, Fibiger HC (1992) Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and anterograde transport and immunohistochemical study. J Comp Neurol 323:387–410
Siegel JM (1979) Behavioral functions of the reticular formation. Brain Res Rev 1:69–105
Siegel JM (2004) Hypocretin (orexin): role in normal behavior and neuropathology. Ann Rev Psychol 55:125–148
Sinnamon HM (1993) Preoptic and hypothalamic neurons and initiation of locomotion in the anesthetized rat. Prog Neurobiol 41:323–344
Spann BM, Grofova I (1991) Nigro-pedunculopontine projection in the rat: an anterograde tracing study with Phaseolus vulgaris-leucoagglutinin (PHA-L). J Comp Neurol 311:375–388
Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, Pierantozzi D, Brusa L, Scarnati E, Mazzone P (2007) Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain 13:1596–1607
Takakusaki K (2008) Forebrain control of locomotor behaviors. Brain Res Rev 57:192–198
Takakusaki K, Habaguchi T, Saitoh K, Kohyama J (2004a) Changes in the excitability of hindlimb motoneurons during muscular atonia induced by stimulating the pedunculopontine tegmental nucleus in cats. Neuroscience 124:467–480
Takakusaki K, Habaguchi T, Ohtinata-Sugimoto J, Saitoh K, Sakamoto T (2003a) Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion; a new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience 119:293–308
Takakusaki K, Kohyama J, Matsuyama K (2003b) Medullary reticulospinal tract mediating a generalized motor inhibition in cats: III. Functional organization of spinal interneurons in the lower lumbar segments. Neuroscience 121:731–746
Takakusaki K, Kohyama J, Matsuyama K, Mori S (1993) Synaptic mechanisms acting on lumbar motoneurons during postural augmentation induced by serotonin injection into the rostral pontine reticular formation in decerebrate cats. Exp Brain Res 93:471–482
Takakusaki K, Kohyama J, Matsuyama K, Mori S (2001) Medullary reticulospinal tract mediating the generalized motor inhibition in cats: parallel inhibitory mechanisms acting on motoneurons and on interneuronal transmission in reflex pathways. Neurosci 103:511–527
Takakusaki K, Ohta R, Harada H (2007) Modulation of the excitability of hindlimb motor neurons during fictive locomotion by the basal ganglia efferents to the brainstem in decerebrate cats. Soc Neurosci Abstr 924.4/PP22
Takakusaki K, Saitoh K, Harada H, Kashiwayanagi M (2004b) Role of basal ganglia – brainstem pathways in the control of motor behaviors. Neurosci Res 50:137–151
Takakusaki K, Saitoh K, Harada H, Kashiwayanagi, M (2006a) The pedunculopontine nucleus and the basal ganglia in locomotion. In: Bezard E (ed) Recent breakthroughs in basal ganglia research. Nova Science Publishing, New York, pp 133–149
Takakusaki K, Saitoh K, Nonaka S, Okumura T, Miyokawa N, Koyama Y (2006b) Neurobiological basis of state-dependent control of motor behavior. Sleep Biol Rhyth 4:87–104
Takakusaki K, Saitoh K, Harada H, Okumura T, Sakamoto T (2004c) Evidence for a role of basal ganglia in the regulation of rapid eye movement sleep by electrical and chemical stimulation for the pedunculopontine tegmental nucleus and the substantia nigra pars reticulata in decerebrate cats. Neuroscience 124:207–220
Takakusaki K, Shimoda N, Matsuyama K, Mori S (1994) Discharge properties of medullary reticulospinal neurons during postural changes induced by intrapontine injections of carbachol, atropine and serotonin, and their functional linkages to hindlimb motoneurons in cats. Exp Brain Res 99:361–374
Takakusaki K, Takahashi K, Saitoh K, Harada H, Okumura T, Kayama Y, Koyama Y (2005) Orexinergic projections to the midbrain mediate alternation of emotional behavioral states from locomotion to cataplexy. J Physiol 568:1003–1020
Thannickal TC, Lai YY, Siegel JM (2007) Hypocretin (orexin) cell loss in Parkinson’s disease. Brain 130:1586–1595
Tomita N, Yano M (2007) Bipedal robot controlled by the basal ganglia and brainstem systems adjusting to indefinite environment. Proc 2007 IEEE/ICME, pp 116–121
Villablanca J, Marcus R (1972) Sleep-wakefulness, EEG and behavioral studies of chronic cats without neocortex and striatum: the ‘diencephalic’ cat. Arch Ital Biol 110:348–382
Villablanca JR, Olmstead CE (1982) The striatum: a fine tuner of the brain. Acta Neurobiol Exp 42:227–299
Yasui K, Inoue Y, Kanbayashi T, Nomura T, Kusumi M, Nakashima K (2006) CSF orexin levels of Parkinson’s disease, dementia with Lewy bodies, progressive supranuclear palsy and corticobasal degeneration. J Neurol Sci 250:120–123
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takakusaki, K., Tomita, N. & Yano, M. Substrates for normal gait and pathophysiology of gait disturbances with respect to the basal ganglia dysfunction. J Neurol 255 (Suppl 4), 19–29 (2008). https://doi.org/10.1007/s00415-008-4004-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-008-4004-7