Abstract
Objective
To quantify spinal cord atrophy and its impact on clinical disability in spinocerebellar ataxia (SCA) type 3 and 6.
Methods
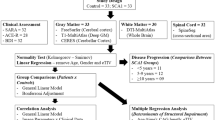
Atrophy of the upper spinal cord was assessed by high resolution T1-weighted MRI of patients with SCA3 (n = 14) and SCA6 (n = 10). Furthermore, two groups of age- and sex-matched healthy control subjects (n = 24,) corresponding to the two SCA groups, were studied. Images were post-processed by a semi-automated volumetry method combining a marker based segmentation and an automatic histogram method facilitating highly reliable quantification and morphometry of the upper cervical cord in vivo.
Results
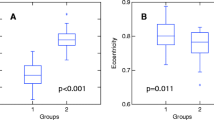
We found a significant reduction of normalized mean crosssectional area of the spinal cord in SCA3 (p < 0.0005), whereas in SCA6 patients normalized mean crosssectional area was in the normal range (p = 0.379). No correlation was found between spinal cord atrophy and disease duration as well as CAG repeat length in both subtypes. In SCA6 a negative dependency between clinical disability, as expressed by the International Cooperative Ataxia Rating Scale as a well established ataxia score, and the mean cross-sectional area was found (p = 0.02). A similar correlation was observed in SCA3 but did not reach statistical significance.
Conclusion
Our results quantify for the first time in vivo spinal cord atrophy as a non-cerebellar neurodegenerative process in SCA3. Our results suggest MR volumetry of the upper cervical cord as a marker of functional importance in SCA3 and SCA6.
Similar content being viewed by others
References
Bakshi R, Dandamudi VS, Neema M, De C, Bermel RA (2005) Measurement of brain and spinal cord atrophy by magnetic resonance imaging as a tool to monitor multiple sclerosis. J Neuroimaging 15:30S–45S
De Michele G, Di Salle F, Filla A, D’Alessio G, Ambrosio G, Viscardi L, Scala R, Campanella G (1995) Magnetic resonance imaging in "typical" and "late onset" Friedreich’s disease and early onset cerebellar ataxia with retained tendon reflexes. Ital J Neurol Sci 16:303–308
Dürr A, Stevanin G, Cancel G, Duyckaerts C, Abbas N, Didierjean O, Chneiweiss H, Benomar A, Lyon-Caen O, Julien J, Serdaru M, Penet C, Agid Y, Brice A (1996) Spinocerebellar ataxia 3 and Machado-Joseph disease: clinical, molecular, and neuropathological features. Ann Neurol 39:490–499
Hahn HK, Millar WS, Klinghammer O, Durkin MS, Tulipano PK, Peitgen HO (2004) A Reliable and Efficient Method for Cerebral Ventricular Volumetry in Pediatric Neuroimaging. Methods Inf Med 43(4):376–382
Klockgether T, Ludtke R, Kramer B, Abele M, Bürk K, Schöls L, Riess O, Laccone F, Boesch S, Lopes-Cendes I, Brice A, Inzelberg R, Zilber N, Dichgans J (1998) The natural history of degenerative ataxia: a retrospective study in 466 patients. Brain 121:589–600
Klockgether T, Skalej M, Wedekind D, Luft AR, Welte D, Schulz JB, Abele M, Bürk K, Laccone F, Brice A, Dichgans J (1998) Autosomal dominant cerebellar ataxia type I. MRI-based volumetry of posterior fossa structures and basal ganglia in spinocerebellar ataxia types 1, 2 and 3. Brain 121:1687–1693
Lukas C, Bellenberg B, Rexilius J, Hahn HK, Kahle M, Köster O, Schimrigk SK (2006) MR-based measurement of spinal cord atrophy in multiple sclerosis: Reproducibility and sensitivity of a new semi-automated procedure. Eur Radiol 16:458
Lukas C, Hahn HK, Bellenberg B, Rexilius J, Schmid G, Schimrigk SK, Przuntek H, Köster O, Peitgen HO (2004) Sensitivity and reproducibility of a new fast 3D segmentation technique for clinical MR-based brain volumetry in multiple sclerosis. Neuroradiology 46:906–915
Mascalchi M, Salvi F, Piacentini S, Bartolozzi C (1994) Friedreich’s ataxia: MR findings involving the cervical portion of the spinal cord. AJR Am J Roentgenol 163:187–191
Miller DH (2004) Biomarkers and surrogate outcomes in neurodegenerative disease: lessons from multiple sclerosis. NeuroRx 1:284–294
Munoz E, Rey MJ, Mila M, Cardozo A, Ribalta T, Tolosa E, Ferrer I (2002) Intranuclear inclusions, neuronal loss and CAG mosaicism in two patients with Machado-Joseph disease. J Neurol Sci 200:19–25
Murata Y, Kawakami H, Yamaguchi S, Nishimura M, Kohriyama T, Ishizaki F, Matsuyama Z, Mimori Y, Nakamura S (1998) Characteristic magnetic resonance imaging findings in spinocerebellar ataxia 6. Arch Neurol 55:1348–1352
Murata Y, Yamaguchi S, Kawakami H, Imon Y, Maruyama H, Sakai T, Kazuta T, Ohtake T, Nishimura M, Saida T, Chiba S, Oh-i T, Nakamura S (1998) Characteristic magnetic resonance imaging findings in Machado-Joseph disease. Arch Neurol 55:33–37
Rashid W, Davies GR, Chard DT, Griffin CM, Altmann DR, Gordon R, Thompson AJ, Miller DH (2006) Increasing cord atrophy in early relapsing-remitting multiple sclerosis: a 3 year study. J Neurol Neurosurg Psychiatry 77:51–55
Satoh JI, Tokumoto H, Yukitake M, Matsui M, Matsuyama Z, Kawakami H, Nakamura S, Kuroda Y (1998) Spinocerebellar ataxia type 6: MRI of three Japanese patients. Neuroradiology 40:222–227
Schmitz-Hübsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, Giunti P, Globas C, Infante J, Kang JS, Kremer B, Mariotti C, Melegh B, Pandolfo M, Rakowicz M, Ribai P, Rola R, Schols L, Szymanski S, van de Warrenburg BP, Dürr A, Klockgether T (2006) Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 66:1717–1720
Schöls L, Amoiridis G, Büttner T, Przuntek H, Epplen JT, Riess O (1997) Autosomal dominant cerebellar ataxia: phenotypic differences in genetically defined subtypes? Ann Neurol 42:924–932
Schöls L, Bauer P, Schmidt T, Schulte T, Riess O (2004) Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol 3:291–304
Takiyama Y, Oyanagi S, Kawashima S, Sakamoto H, Saito K, Yoshida M, Tsuji S, Mizuno Y, Nishizawa M (1994) A clinical and pathologic study of a large Japanese family with Machado-Joseph disease tightly linked to the DNA markers on chromosome 14q. Neurology 44:1302–1308
Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B (1997) International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci 145:205–211
Villanueva-Haba V, Garces-Sanchez M, Bataller L, Palau F, Vilchez J (2001) Neuroimaging study with morphometric analysis of hereditary and idiopathic ataxia. Neurologia 16:105–111
Wessel K, Schroth G, Diener HC, Muller-Forell W, Dichgans J (1989) Significance of MRI-confirmed atrophy of the cranial spinal cord in Friedreich’s ataxia. Eur Arch Psychiatry Neurol Sci 238:225–230
Yoshizawa T, Watanabe M, Frusho K, Shoji S (2003) Magnetic resonance imaging demonstrates differential atrophy of pontine base and tegmentum in Machado-Joseph disease. J Neurol Sci 215:45–50
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lukas, C., Hahn, H.K., Bellenberg, B. et al. Spinal cord atrophy in spinocerebellar ataxia type 3 and 6. J Neurol 255, 1244–1249 (2008). https://doi.org/10.1007/s00415-008-0907-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-008-0907-6