Abstract
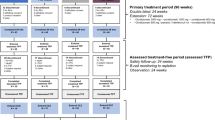
We aimed to evaluate the safety and MRI efficacy of interferon beta-1b (IFNβ-1b) 375 μg (subcutaneously [sc] every other day [eod]) in relapsing-remitting multiple sclerosis (RRMS) patients with a suboptimal response to IFNβ-1b 250 μg, i.e., with MRI activity or relapses. The OPTimization of Interferon for MS (OPTIMS) study was a prospective multicenter randomized phase 2 trial comprising a 6-month run-in phase (to identify suboptimal responders) and a 6-month randomized phase of open-label clinical and blinded MRI follow-up. During run-in all patients were treated with IFNβ-1b 250 μg sc eod; during the study phase suboptimal treatment responders were randomized either to IFNβ-1b 250 or 375 μg sc eod. Primary outcome was the proportion of patients without MRI activity during study Months 9–12 according to the intention-totreat principle. 216 RRMS patients entered the study: 83 suboptimal responders were identified and randomized, 7 refused to continue treatment, 76 were included in the analysis. More patients treated with 375 μg had no MRI activity at Months 9–12 (30/36 vs.16/40; relative risk, 0.28; 95 % confidence interval, 0.08–0.47; p = 0.0001). Sensitivity analysis (“worst case scenario”) confirmed the results. No new or unexpected adverse events were observed, but there was a trend towards more withdrawals in the 375 μg group. Increasing the dose of IFNβ-1b from 250 μg to 375 μg is a successful strategy for reducing subclinical signs of disease activity in RRMS patients. Further studies are needed to show whether this dose may also improve clinical efficacy.
Similar content being viewed by others
References
Arnason BG for the BEYOND Study Steering Committee (2005) BEYOND – Betaseron®/Betaferon® efficacy yielding outcomes of a new dose. Neurology 62(Suppl 5):A490. Abstract
Calabresi PA, Stone LA, Bash C (1997) Interferon beta results in immediate reduction of contrast-enhanced MRI lesions in multiple sclerosis patients followed by weekly MRI. Neurology 48:1446–1448
Durelli L, Oggero A, Verdun E, et al. (2000) Does high-dose interferon beta- 1b improve clinical response in more severely disabled multiple sclerosis patients? J Neurol Sci 178:37–41
Durelli L, Verdun E, Barbero P, et al. (2002) Every-other-day interferon beta-1b versus once-weekly interferon beta- 1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN). Lancet 359:1453–1460
Files JG, Gray JL, Do LT (1998) A novel sensitive and selective bioassay for human type I interferons. J Interferon Cytokine Res 18:1019–1024
Goodin DS, Frohman EM, Garmany GP Jr, et al. (2002) Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology 58:169–178
Goodin DS, Frohman EM, Hurwitz B, et al. (2007) Neutralizing antibodies to interferon beta: Assessment of their clinical and radiographic impact: An evidence report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 68:977–984
Kappos L, Moeri D, Radue EW (1999) Predictive value of gadoliniumenhanced magnetic resonance imaging for relapse rate and changes in disability or impairment in multiple sclerosis: a meta-analysis. Gadolinium MRI Meta-analysis Group. Lancet 353:964–969
Khan OA, Tselis AC, Kamholz JA, et al. (2001) A prospective, open-label treatment trial to compare the effect of IFNbeta-1a (Avonex), IFNbeta-1b (Betaseron), and glatiramer acetate (Copaxone) on the relapse rate in relapsing – remitting multiple sclerosis: results after 18 months of therapy. Mult Scler 7:349–353
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444–1452
McFarland HF, Frank JA, Albert PS (1992) Using gadolinium-enhanced magnetic resonance imaging lesions to monitor disease activity in multiple sclerosis. Ann Neurol 32:758–766
Morton V, Torgerson DJ (2005) Regression to the mean: treatment effect without the intervention. J Eval Clin Pract 11:59–65
Panitch H, Goodin DS, Francis G, et al. (2002) Randomized, comparative study of interferon beta-1a treatment regimens in MS: The EVIDENCE Trial. Neurology 59:1496–1506
Poser CM, Paty DW, Scheinberg L, et al. (1983) New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 13:227–231
PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group (1998) Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 352:1498–1504
Pungor E Jr, Files JG, Gabe JD (1998) A novel bioassay for the determination of neutralizing antibodies to IFN-beta- 1b. J Interferon Cytokine Res 18:1025–1030
Quesada J (1992) Toxicity and side effects of interferons. In: Baron S, Coppenhaver D, Dianzani F (eds) Interferon: principles and medical applications. Galveston, TX: The University of Texas Medical Branch at Galveston Department of Microbiology, pp 427–432
Rio J, Nos C, Tintore M (2006) Defining the response to interferon-beta in relapsing-remitting multiple sclerosis patients. Ann Neurol 59:344–352
Rothuizen LE, Buclin T, Spertini F, et al. (1999) Influence of interferon beta-1a dose frequency on PBMC cytokine secretion and biological effect markers. J Neuroimmunol 99:131–141
Rovaris M, Comi G, Ladkani D (2003) Short-term correlations between clinical and MR imaging findings in relapsing- remitting multiple sclerosis. Am J Neuroradiol 24:75–81
Sorensen PS, Tscherning T, Mathiesen HK, et al. (2006) Neutralizing antibodies hamper IFNβ bioactivity and treatment effect on MRI in patients with MS. Neurology 67:1681–1683
Sormani MP, Bruzzi P, Miller DH, Gasperini C, Barkhof F, Filippi M (1999) Modelling MRI enhancing lesion counts in multiple sclerosis using a negative binomial model: implications for clinical trials. J Neurol Sci 163:74–80
Stone LA, Frank JA, Albert PS, et al. (1995) The effect of interferon-beta on blood-brain barrier disruptions demonstrated by contrast-enhanced magnetic resonance imaging in relapsingremitting multiple sclerosis. Ann Neurol 37:611–619
Stone LA, Frank, JA, Albert PS (1997) Characterization of MRI response to treatment with interferon beta-1b: contrast-enhancing MRI lesion frequency as a primary outcome measure. Neurology 49:862–869
The IFNB Multiple Sclerosis Study Group (1993) Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, doubleblind, placebo-controlled trial. Neurology 43:655–661
The IFNB Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group (1995) Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology 45:1277–1285
The Once Weekly Interferon for MS Study Group (1999) Evidence of interferon beta-1a dose response in relapsing- remitting MS: the OWIMS Study. Neurology 53:679–686
Tremlett HL, Oger J (2003) Interrupted therapy: stopping and switching of the beta-interferons prescribed for MS. Neurology 61:551–554
Williams GJ, Witt PL (1998) Comparative study of the pharmacodynamic and pharmacologic effects of Betaseron and AVONEX. J Interferon Cytokine Res 18:967–975
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
*Members of OPTIMS Study Group are listed in the Appendix.
Rights and permissions
About this article
Cite this article
Durelli, L., Barbero, P., Bergui, M. et al. The OPTimization of Interferon for MS Study: 375 μg interferon beta-1b in suboptimal responders. J Neurol 255, 1315–1323 (2008). https://doi.org/10.1007/s00415-008-0879-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-008-0879-6