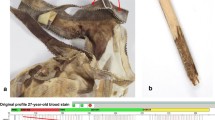
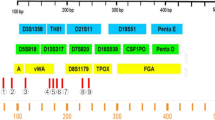
Abstract
Blood stain evidence obtained from a violent crime scene provides decisive clues that can enable a case to be solved through forensic analyses such as genetic identification. However, collected samples usually contain a mixture of biological material from different sources, making genetic identification difficult. To address this issue, we developed an activatable aptamer sensor targeting 17β-estradiol for detection of female-specific blood in mixed samples. With the sensor, we were able to detect blood originating from females using a variable light source (495 nm). The sensor was especially sensitive to blood from young females (10–40 years) but not to blood from older females (≥ 50 years). Genomic DNA was extracted from the female blood specimens identified by this method and used for quantification and short tandem repeat genotyping. We confirmed that there was no fluorescence interference from the aptamer sensor. These results indicate that this novel aptamer sensor can be used to analyze evidentiary blood samples and thereby facilitate subsequent genetic identification.






Similar content being viewed by others
References
Shi H, He X, Wang K, Wu X, Ye X, Guo Q, Tan W, Qing Z, Yang X, Zhou B (2011) Activatable aptamer probe for contrast-enhanced in vivo cancer imaging based on cell membrane protein-triggered conformation alteration. Proc Natl Acad Sci U S A 108:3900–3905. https://doi.org/10.1073/pnas.1016197108
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–822. https://doi.org/10.1038/346818a0
Daniels DA, Chen H, Hicke BJ, Swiderek KM, Gold L (2003) Atenascin-C aptamer identified by tumor cell SELEX: systematic evolution of ligands by exponential enrichment. Proc Natl Acad Sci U S A 100:15416–15421. https://doi.org/10.1073/pnas.2136683100
Liu J, Bai W, Niu S, Zhu C, Yang S, Chen A (2014) Highly sensitive colorimetric detection of 17beta-estradiol using split DNA aptamers immobilized on unmodified gold nanoparticles. Sci Rep 4:7571. https://doi.org/10.1038/srep07571
Stoltenburg R, Reinemann C, Strehlitz B (2007) SELEX—a (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol Eng 24:381–403. https://doi.org/10.1016/j.bioeng.2007.06.001
McKeague M, Derosa MC (2012) Challenges and opportunities for small molecule aptamer development. J Nucleic Acids 2012:748913. https://doi.org/10.1155/2012/748913
Shi H, Tang Z, Kim Y, Nie H, Huang YF, He X, Deng K, Wang K, Tan W (2010) In vivo fluorescence imaging of tumors using molecular aptamers generated by cell-SELEX. Chem Asian J 5:2209–2213. https://doi.org/10.1002/asia.201000242
Huang P, Chandra V, Rastinejad F (2010) Structural overview of the nuclear receptor superfamily: insights into physiology and therapeutics. Annu Rev Physiol 72:247–272. https://doi.org/10.1146/annurev-physiol-021909-135917
Bondar G, Kuo J, Hamid N, Micevych P (2009) Estradiol-induced estrogen receptor-alpha trafficking. J Neurosci 29:15323–15330. https://doi.org/10.1523/JNEUROSCI.2107-09.2009
Ryan KJ (1982) Biochemistry of aromatase: significance to female reproductive physiology. Cancer Res 42: 3342s–3344s
Tai SS, Welch MJ (2005) Development and evaluation of a reference measurement procedure for the determination of estradiol-17beta in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem 77:6359–6363. https://doi.org/10.1021/ac050837i
Yildirim N, Long F, Gao C, He M, Shi HC, Gu AZ (2012) Aptamer-based optical biosensor for rapid and sensitive detection of 17beta-estradiol in water samples. Environ Sci Technol 46:3288–3294. https://doi.org/10.1021/es203624w
Huh YS, Erickson D (2010) Aptamer based surface enhanced Raman scattering detection of vasopressin using multilayer nanotube arrays. Biosens Bioelectron 25:1240–1243. https://doi.org/10.1016/j.bios.2009.09.040
Chen JW, Liu XP, Feng KJ, Liang Y, Jiang JH, Shen GL, Yu RQ (2008) Detection of adenosine using surface-enhanced Raman scattering based on structure-switching signaling aptamer. Biosens Bioelectron 24:66–71. https://doi.org/10.1016/j.bios.2008.03.013
Liu J, Cao Z, Lu Y (2009) Functional nucleic acid sensors. Chem Rev 109:1948–1998. https://doi.org/10.1021/cr030183i
Kim YS, Jung HS, Matsuura T, Lee HY, Kawai T, MB G (2007) Electrochemical detection of 17beta-estradiol using DNA aptamer immobilized gold electrode chip. Biosens Bioelectron 22:2525–2531. https://doi.org/10.1016/j.bios.2006.10.004
Alsager OA, Kumar S, Zhu B, Travas-Sejdic J, McNatty KP, Hodgkiss JM (2015) Ultrasensitive colorimetric detection of 17beta-estradiol: the effect of shortening DNA aptamer sequences. Anal Chem 87:4201–4209. https://doi.org/10.1021/acs.analchem.5b00335
Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31:3406–3415. https://doi.org/10.1093/nar/gkg595
Lee H, Park MJ, Sun SH, Choi DH, Lee YH, Park KW, Chun BW (2016) Ascorbic acid and vitamin C-containing beverages delay the leucomalachite green reaction to detect latent bloodstains. Leg Med (Tokyo) 23:79–85. https://doi.org/10.1016/j.legalmed.2016.10.003
Stoica BA, Bunescu S, Neamtu A, Bulgaru-Iliescu D, Foia L, Botnariu EG (2016) Improving luminol blood detection in forensics. J Forensic Sci 61:1331–1336. https://doi.org/10.1111/1556-4029.13141
Quickenden TI, Cooper PD (2001) Increasing the specificity of the forensic luminol test for blood. Luminescence 16:251–253. https://doi.org/10.1002/bio.635
Quickenden TI, Creamer JI (2001) A study of common interferences with the forensic luminol test for blood. Luminescence 16:295–298. https://doi.org/10.1002/bio.657
Gao W, Wang C, Muzyka K, Kitte SA, Li J, Zhang W, Xu G (2017) Artemisinin-luminol chemiluminescence for forensic bloodstain detection using a smart phone as a detector. Anal Chem 89:6160–6165. https://doi.org/10.1021/acs.analchem.7b01000
Ermida C, Navega D, Cunha E (2017) Luminol chemiluminescence: contribution to postmortem interval determination of skeletonized remains in Portuguese forensic context. Int J Legal Med 131:1149–1153. https://doi.org/10.1007/s00414-017-1547-0
Caudullo G, Caruso V, Cappella A, Sguazza E, Mazzarelli D, Amadasi A, Cattaneo C (2017) Luminol testing in detecting modern human skeletal remains: a test on different types of bone tissue and a caveat for PMI interpretation. Int J Legal Med 131:287–292. https://doi.org/10.1007/s00414-016-1493-2
Blenkharn JI (2008) Luminol-based forensic detection of latent blood; an approach to rapid wide-area screening combined with Glo-Germ oil simulant studies. J Hosp Infect 69:405–406. https://doi.org/10.1016/j.jhin.2008.04.006
Lakhin AV, Tarantul VZ, Gening LV (2013) Aptamers: problems, solutions and prospects. Acta Nat 5:34–43
Mayer G (2009) The chemical biology of aptamers. Angew Chem Int Ed Engl 48:2672–2689. https://doi.org/10.1002/anie.200804643
Strehlitz B, Reinemann C, Linkorn S, Stoltenburg R (2012) Aptamers for pharmaceuticals and their application in environmental analytics. Bioanal Rev 4:1–30. https://doi.org/10.1007/s12566-011-0026-1
Yoshida W, Mochizuki E, Takase M, Hasegawa H, Morita Y, Yamazaki H, Sode K, Ikebukuro K (2009) Selection of DNA aptamers against insulin and construction of an aptameric enzyme subunit for insulin sensing. Biosens Bioelectron 24:1116–1120. https://doi.org/10.1016/j.bios.2008.06.016
Greenblatt RB, Oettinger M, Bohler CS (1976) Estrogen-androgen levels in aging men and women: therapeutic considerations. J Am Geriatr Soc 24:173–178. https://doi.org/10.1111/j.1532-5415.1976.tb04294.x
Rettberg JR, Yao J, Brinton RD (2014) Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol 35:8–30. https://doi.org/10.1016/j.yfrne.2013.08.001
Funding
This research was supported by the Korean government and by a grant from the Forensic Research Program of the National Forensic Service (no. NFS2017DNA02).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
ESM 1
(PDF 563 kb).
Rights and permissions
About this article
Cite this article
Kim, JY., Park, JH., Kim, M.I. et al. Identification of female-specific blood stains using a 17β-estradiol-targeted aptamer-based sensor. Int J Legal Med 132, 91–98 (2018). https://doi.org/10.1007/s00414-017-1718-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-017-1718-z