Abstract
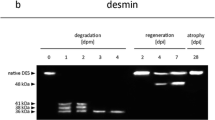
While the determination of postmortem interval (PMI) is a crucial and fundamental step in any death investigation, the development of appropriate biochemical methods for PMI estimation is still in its infancy. This study focused on the temperature-dependent postmortem degradation of calcineurin A (CnA), calmodulin-dependent kinase II (CaMKII), myristoylated alanine-rich C-kinase substrate (MARCKs), and protein phosphatase 2A (PP2A) in mice. The results show that MARCKS, CaMKII, and the use of lung tissue do not appear to warrant further study for the determination of PMI in humans. In skeletal muscle, CnA underwent a rapid temperature-dependent cleavage (60 → 57 kDa) over the first 48 h of postmortem interval. At 21°C, this transformation was completed within 24 h. In contrast, PP2A increased within the first 24 h after which it degraded at 21°C but remained stable for up to 96 h at 5°C and 10°C. The 60 → 57 kDa postmortem conversion of CnA was inhibited by addition of protease inhibitors and MDL-28170 indicating a calpain pathway mediates this breakdown. Proteasome inhibition (MG-132) and calmodulin antagonism (calmidazolium) also inhibited this conversion suggesting that other protein degradation pathways also are in play. In contrast, all of the protease inhibitors and calmidazolium but not ethylene glycol tetraacetic acid led to increased levels of PP2A. The data are discussed in terms of developing a useable field-based biochemical assay for postmortem interval determination in humans and understanding the protein degradation pathways that are initiated upon death.







Similar content being viewed by others
References
Pounder DJ (1995) Postmortem changes and time of death. Department of Forensic Medicine, University of Dunde, Dunde
Hayashi T, Ishida Y, Mizunuma S, Kimura A, Kondo T (2008) Differential diagnosis between freshwater drowning and saltwater drowning based on intrapulmonary aquaporin-5 expression. Int J Legal Med 123:7–13
Kang S, Kassam N, Gauthier ML, O’Day DH (2003) Post-mortem changes in calmodulin binding proteins in muscle and lung. Forensic Sci Int 131:140–147
Bauer M, Gramlich I, Polzin S, Patzelt D (2003) Quantification of mRNA degradation as possible indicator of postmortem interval-a pilot study. Legal Med 5:220–227
Yi S-H, Zhao X-H, Liu L (2008) Selection of parameters to infer postmortem interval by detecting DNA degradation using comet assay. Chinese J Forensic Med 23:1–4
Tao T, Xu J, Luo T-X, Liao Z-G, Pan H-F (2006) Contents of vitreous humor of dead body with different postmortem intervals. J Sichuan Univ 37:898–900
Wehner F, Wehner HD, Schieffer MC, Subke J (1999) Delimitation of the time of death by immunohistochemical detection of insulin in pancreatic β-cells. Forensic Sci Int 105:161–169
Neis P, Hille R, Paschke M, Pilwat G, Schnabel A, Neiss C, Bratzke H (1999) Strontium90 for determination of time since death. Forensic Sci Int 99:47–51
Mittmeyer HJ (1980) Determination of myo-albumin content. Z Rechtsmed 84:233–237
Takeichim S, Tokunaga I, Yoshima K, Maeiwa M, Bando Y, Kominami E, Katunuma N (1984) Mechanism of postmortem autolysis of skeletal muscle. Biochem Med 32:341–348
Verkhratsky A (2007) Calcium and cell death. Subcell Biochem 45:465–480
Cohen P, Klee CB (1988) Calmodulin. Elsevier, Amsterdam
Geesink GH, Kuchay S, Chishti AH, Koohmaraie M (2006) μ-Calpain is essential for postmortem proteolysis of muscle proteins. J Anim Sci 84:2834–2840
Sorimachi Y, Harada K, Yoshida K-I (1996) Involvement of calpain in postmortem proteolysis in the rat brain. Forensic Sci Int 81:165–174
Geesink GH, Koohmaraie M (1999) Effect of calpastatin on degradation of myofibrillar proteins by μ-calpain under postmortem conditions. J Anim Sci 77:2685–2692
Johnson GVW, Greenwood JA, Costello AC, Troncoso JC (1991) The regulatory role of calmodulin in the proteolysis of individual neurofilament proteins by calpain. Neurochem Res 16:869–873
Morioka M, Hamada J-I, Ushio Y, Miyamoto E (1999) Potential role of calcineurin for brain ischemia and traumatic injury. Prog Neurobiol 58:1–30
Wu H-Y, Tomizawa K, Oda Y et al (2004) Critical role of calpain-mediated cleavage of calcineurin in excitotoxic neurodegeneration. J Biol Chem 279:4929–44940
Shibasaki F, McKeon F (1995) Calcineurin functions in Ca (2+)-activated cell death in mammalian cells. J Cell Biol 131:735–743
Liu F, Grundke-Iqbal I, Iqbal K, Oda Y, Tomizawa K, Gong C-X (2005) Truncation and activation of calcineurin A by calpain I in Alzheimer disease brain. J Biol Chem 280:37755–37762
Shioda N, Moriguchi S, Shirasaki Y, Fukunaga K (2006) Generation of constitutively active calcineurin by calpain contributes to delayed neuronal death following mouse brain ischemia. J Neurochem 98:310–320
Taniguchi S, Fujita Y, Hayashi S et al (2001) Calpain-mediated degradation of p35 to p25 in postmortem human and rat brains. FEBS Lett 489:46–50
Sanoudou D, Kang PB, Haslett JN, Han M, Kunkel LM, Beggs AH (2004) Transcriptional profile of postmortem skeletal muscle. Physiol Genomics 16:222–228
Fountoulakis M, Hardmeier R, Hoger H, Lubec G (2001) Postmortem changes in the level of brain protein. Exp Neurol 167:86–91
Geesink GH, Koohmaraie M (1999) Postmortem proteolysis and calpain/calpastatin activity in callipyge and normal lamb biceps femoris during extended postmortem storage. J Anim Sci 77:1490–1501
Sabucedo AJ, Furton KG (2003) Estimation of postmortem interval using the protein cardiac troponin I. Forensic Sci Int 134:11–16
Xiao JH, Chen YC (2005) A study on the postmortem relationship between the degradation of protein and the postmortem interval. Fa Yi Xue Za Zhi 21:110–112
Liu Y, Kuai J-X, Zhang Y-W, Wang Y-Y (2008) The relationship between the degradation of actin and the postmortem interval in rats. J Forensic Med 24:165–167
Kuai J-X, Liu Y, Zhang Y-W (2008) A study on the relationship between the degradation of tubulin in cardiac muscle and lung of rat and the postmortem interval. Chinese J Forensic Med 23:96–98
Kitamura N, Nishino N, Hashimoto T et al (1998) Asymmetrical changes in the fodrin α subunit in the superior temporal cortices in schizophrenia. Biol Psychiatry 43:254–262
Li J, Gould TD, Yuan P, Manji HK, Chen G (2003) Post-mortem interval effects on the phosphorylation of signaling proteins. Neuropsychopharmacology 28:1017–1025
Zolk O, Schenke C, Sarikas A (2006) The ubiquitin-proteasome system: focus on the heart. Cardiovasc Res 70:410–421
Rastogi S, Sentex E, Elimban V, Dhalla NS, Netticadan T (2003) Elevated levels of protein phosphatase 1 and phosphatase 2A may contribute to cardiac dysfunction in diabetes. Biochim Biophys Acta 1638:271–277
Althaus L, Stuckradt S, Henbge C, Bajanowski T (2007) Cooling experiments using dummies covered by leaves. Int J Legal Med 121:112–114
Acknowledgments
We thank Andrew Catalano for his assistance with the dissection, Xavier D’Souza for assistance with animal care and maintenance, and Michael D. Rennie for assistance with the statistics. This project was supported by a grant from the Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poloz, Y.O., O’Day, D.H. Determining time of death: temperature-dependent postmortem changes in calcineurin A, MARCKS, CaMKII, and protein phosphatase 2A in mouse. Int J Legal Med 123, 305–314 (2009). https://doi.org/10.1007/s00414-009-0343-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-009-0343-x