Abstract
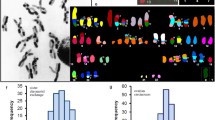
The structure of human metaphase chromosomes, fixed according to standard procedures for optical microscopy but not treated for banding, was exammed by atomic force microscopy (AFM). The images show that chromosomes display a banding pattern very similar to G-banding, detected by the AFM as a variation in the thickness of chromatin. This similarity allows the identification of individual chromosomes.
Similar content being viewed by others
References
Allegrini M, Arpa E, Ascoli C, Baschieri P, Dinelli F, Frediani C, Labardi M, Lio A, Mariani T, Vanni L (1993) Scanning probe microscope with interchangeable AFM-FFM and STM heads. II Nuovo Cimento 15D:279–292
Ambros PF, Sumner AT (1987) Correlation of pachytene chromomeres and metaphase bands of human chromosomes and distinctive properties of telomeric regions. Cytogenet Cell Genet 44: 223–228
Bahr GF, Larsen PM (1974) Structural bands in human chromosomes. Adv Cell Mol Biol 3: 191–212
Bath DW (1976) Surface ultrastructure of trypsin-banded chromosomes. Exp Cell Res 98: 262–268
Bernardi G (1989) The isochore organization of the human genome. Annu Rev Genet 23: 637–661
Bickmore WA, Sumner AT (1989) Mammalian chromosome banding-an expression of genome organization. Trends Genet 5: 144–148
Binning G, Quate CF, Gerber CH (1986) Atomic force microscope. Phys Rev Lett 56: 930–933
Bird AP (1986) CpC-rich islands and function of DNA methylation. Nature 321: 209–213
Bird AP (1987) CpG islands as gene markers in the vertebrate nucleus. Trends Genet 3: 342–347
Burkholder GD (1974) Electron microscopic visualization of chromosomes banded with trypsin. Nature 247: 292–294
Burkholder GD (1975) The ultrastructure of G and C band chromosomes. Exp Cell Res 90: 269–278
Burkholder GD, Duczek LL (1982) The effect of chromosome banding techniques on the proteins of isolated chromosomes. Chromosoma 87: 425–435
Butt HJ, Wolff EK, Gould SAC, Dixon Northern B, Peterson CM, Hasma PK (1991) Imaging cells with the atomic force microscope. J Struct Biol 105: 54–61
Comings DE (1978) Mechanism of chromosome banding and implications for chromosome structure. Annu Rev Genet 12: 25–46
Craig JM, Bickmore WA (1993) Chromosome bands — Flavours to savour. BioEssay 15: 349–354
De Grooth BG, Putman CAJ (1992) High-resolution imaging of chromosomes related structures by atomic force microscopy. J Microsc 168: 239–247
FangJS, Jagiello GM (1988) An analysis of the chromomere map and chiasmata characteristics of human diplotene spermatocytes. Cytogenet Cell Genet 47: 52–57
Gessler M, Bruns GAP (1989) A physical map around the WAGR complex on the short arm of chromosome 11. Genomics 5: 43–55
Goldman MA, Holmquist GP, Gray MC, Caston LA, Nag A (1984) Replication timing of genes and middle repetitive sequences. Science 224: 686–692
Gould SAC, Drake B, Prater CB, Weisenhorn AL, Manne S, Hansma HG, Hansma PK, Massie J, Longmire M, Elings V, Dixon Northern B, Murkergee B, Peterson CM, Stoeckenius W, Albrecht TR, Quate CF (1990) From atoms to integrated circuit chips, blood cells and bacteria with atomic force microscope. J Vac Sci Technol A8: 369–372
Harrison CJ, Britch M, Allen TD, Harris R (1981) Scanning electron microscopy of G-banded human karyotype. Exp Cell Res 134: 141–153
Heneen WK, Caspersson T (1973) Identification of chromosomes of rye by distribution patterns of DNA. Hereditas 74: 259–272
Hoh JH, Hansma PK (1992) Atomic force microscopy for high-resolution imaging in cell biology. Trends Cell Biol 2: 208–213
Holmquist GP (1987) Role of replication time in the control of tissue-specific gene expression. Am J Hum Genet 40: 151–173
Holmquist GP (1992) Chromosome bands, their chromatin flavors and their functional features. Am J Hum Genet 51: 17–37
Holmquist GP, Gray M, Porter T, Jordan J (1982) Characterization of Giemsa dark and light band DNA. Cell 31: 121–129
Korenberg JR, Rykowski MC (1988) Human genome organization: Alu, Lines, and molecular structure of metaphase chromosome bands. Cell 53: 391–400
Manuelidis L, Ward DC (1984) Chromosomal and nuclear distribution of the Hind III 1.9 kb human DNA repeat segment. Chromosoma 91: 28–38
McKay RDG (1973) The mechanism of G and C banding in mammalian metaphase chromosomes. Chromosoma 44: 1–14
Mezzanotte R, Vanni R, Flore O, Ferrucci L, Sumner T (1988) Ageing of fixed cytological preparations produces degradation of chromosomal DNA. Cytogenet Cell Genet 48: 60–62
Okada TA, Comings DE (1974) Mechanism of chromosome banding III. Similarity between G-bands of mitotic chromosomes and chromomeres of meiotic chromosomes. Chromosoma 48: 65–71
Putman CAJ, Van der Werf KO, De Grooth BG, Van Hulst NF, Segerink FB, Greve J (1992) Atomic force microscope featuring an integrated optical microscope. Ultramicroscopy 42/44: 1549–1552
Saccone S, De Sario A, Della Valle G, Bernardi G (1992) The highest gene concentrations in the human genome are in telomeric bands of metaphase chromosomes. Proc Natl Acad Sci 89: 4913–4917
Sumner AT, Evans HJ, Buckland RA (1973) Mechanism involved in the banding of chromosomes with quinacrine and Giemsa. Exp Cell Res 81: 214–222
Yunis JJ, Sanchez O (1973) G-banding and chromosome structure. Chromosoma 44: 15–23
Zatsepina OV, Polyakov VY, Chentsov YS (1989) Differential decondensation of mitotic chromosomes during hypotonic treatment of living cells as a possible cause of G-banding: an ultrastructural study. Chromosoma 98: 109–116
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Musio, A., Mariani, T., Frediani, C. et al. Longitudinal patterns similar to G-banding in untreated human chromosomes: evidence from atomic force microscopy. Chromosoma 103, 225–229 (1994). https://doi.org/10.1007/BF00368016
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00368016