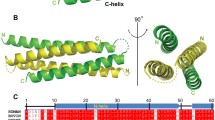
Abstract
In most eukaryotes, homologous chromosomes (homologs) are closely apposed during the prophase of the first meiotic division by a ladderlike proteinaceous structure, the synaptonemal complex (SC) [Fawcett, J Biophys Biochem Cytol 2:403–406, 1956; Moses, J Biophys Biochem Cytol 2:215–218, 1956]. SCs consist of two proteinaceous axes, which each support the two sister chromatids of one homolog, and numerous transverse filaments (TFs), which connect the two axes. Organisms that assemble SCs perform meiotic recombination in the context of these structures. Although much information has accumulated about the composition of SCs and the pathways of meiotic crossing over, several questions remain about the role of SCs in meiosis, in particular, about the role of the TFs. In this review, we focus on possible role(s) of TFs. The interest in TF functions received new impulses from the recent characterization of TF-deficient mutants in a number of species. Intriguingly, the phenotypes of these mutants are very different, and a variety of TF functions appear to be hidden behind a façade of morphological conservation. However, in all TF-deficient mutants a specific class of crossovers that display interference is affected. TFs appear to create suitable preconditions for the formation of these crossovers in most species, but are most likely not directly involved in the interference process itself. Furthermore, TFs are important for full-length homolog alignment.



Similar content being viewed by others
References
Agarwal S, Roeder GS (2000) Zip3 provides a link between recombination enzymes and synaptonemal complex proteins. Cell 102:245–255
Alpi A, Pasierbek P, Gartner A, Loidl J (2003) Genetic and cytological characterization of the recombination protein RAD-51 in Caenorhabditis elegans. Chromosoma 112:6–16
Anderson LK, Offenberg HH, Verkuijlen WC, Heyting C (1997) RecA-like proteins make part of early meiotic nodules in lily. Proc Natl Acad Sci U S A 94:6868–6873
Anderson LK, Doyle GG, Brigham B, Carter J, Hooker KD, Lai A, Rice M, Stack SM (2003) High-resolution crossover maps for each bivalent of Zea mays using recombination nodules. Genetics 165:849–865
Anderson LK, Royer SM, Page SL, McKim KS, Lai A, Lilly MA, Hawley RS (2005) Juxtaposition of C(2)M and the transverse filament protein C(3)G within the central region of Drosophila synaptonemal complex. Proc Natl Acad Sci U S A 102:4482–4487
Anderson LK, Stack SM (2005) Recombination nodules in plants. Cytogenet Genome Res 109:198–204
Argueso JL, Wanat J, Gemici Z, Alani E (2004) Competing crossover pathways act during meiosis in Saccharomyces cerevisiae. Genetics 168:1805–1816
Ashley T, Plug AW (1998) Caught in the act: deducing meiotic function from protein immunolocalization. Curr Top Dev Biol 37:201–239
Baker SM, Plug AW, Prolla TA, Bronner CE, Harris AC, Yao X, Christie D-M, Monell C, Arnheim N, Bradley A, Ashley T, Liskay RM (1996) Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat Genet 13:336–342
Baudat F, Manova K, Yuen JP, Jasin M, Keeney S (2000) Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol Cell 6:989–998
Bellani MA, Romanienko PJ, Cairatti DA, Camerini-Otero RD (2005) SPO11 is required for sex-body formation, and Spo11 heterozygosity rescues the prophase arrest of Atm−/− spermatocytes. J Cell Sci 118:3233–3245
Bishop D (1994) RecA homologs Dmc1 and Rad51 interact to form multiple nuclear complexes prior to meiotic chromosome synapsis. Cell 79:1081–1092
Bishop DK, Zickler D (2004) Early decision: Meiotic crossover interference prior to stable strand exchange and synapsis. Cell 117:9–15
Bogdanov YF (2003) Variation and evolution of meiosis. Russ J Genet 39:363–381
Bogdanov YF, Grishaeva TM, Dadashev SY (2002) Gene CG17604 of Drosophila melanogaster may be a functional homolog of yeast gene ZIP1 and mammal gene SCP1 (SYCP1) encoding proteins of the synaptonemal complex. Russ J Genet 38:90–94
Börner GV, Kleckner N, Hunter N (2004) Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell 117:29–45
Broman KW, Rowe LB, Churchill GA, Paigen K (2002) Crossover interference in the mouse. Genetics 160:1123–1131
Burgess SM (2004) The ends are the means. Mol Cell 13:766–768
Cao L, Alani E, Kleckner N (1990) A pathway for generation and processing of double-strand breaks during meiotic recombination in S. cerevisiae. Cell 61:1089–1101
Carpenter ATC (1987) Gene conversion, recombination nodules, and the initiation of meiotic synapsis. Bioessays 6:232–236
Chen C, Zhang W, Timofejeva L, Gerardin Y, Ma H (2005) The Arabidopsis ROCK-N-ROLLERS gene encodes a homolog of the yeast ATP-dependent DNA helicase MER3 and is required for normal meiotic crossover formation. Plant J 43:321–334
Chua PR, Roeder GS (1997) Tam1, a telomere associated meiotic protein, functions in chromosome synapsis and crossover interference. Genes Dev 11:1786–1800
Chua PR, Roeder GS (1998) Zip2, a meiosis-specific protein required for the initiation of chromosome synapsis. Cell 93:349–359
Colaiácovo MP, MacQueen AJ, Martinez PE, McDonald K, Adamo A, La Volpe A, Villeneuve AM (2003) Synaptonemal complex assembly in C. elegans is dispensable for loading strand-exchange proteins but critical for proper completion of recombination. Dev Cell 5:463–474
Conrad MN, Dominguez AM, Dresser ME (1997) Ndj1p, a meiotic telomere protein required for normal chromosome synapsis and segregation in yeast. Science 276:1252–1255
Copenhaver GP, Housworth EA, Stahl FW (2002) Crossover interference in Arabidopsis. Genetics 160:1631–1639
de los Santos T, Hunter N, Lee C, Larkin B, Loidl J, Hollingsworth NM (2003) The Mus81/Mms4 endonuclease acts independently of double-Holliday junction resolution to promote a distinct subset of crossovers during meiosis in budding yeast. Genetics 164:81–94
de Vries FAT, de Boer E, van den Bosch M, Baarends WM, Ooms M, Yuan L, Liu J-G, Heyting C, Pastink A (2005) Mouse Sycp1 functions in synaptonemal complex assembly, meiotic recombination, and XY body formation. Genes Dev 19:1376–1389
Dernburg AF, McDonald K, Moulder G, Barstead R, Dresser M, Villeneuve AM (1998) Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 94:387–398
Dobson M, Pearlman RE, Karaiskakis A, Spyropoulos B, Moens PB (1994) Synaptonemal complex proteins: occurrence, epitope mapping, and chromosome disjunction. J Cell Sci 107:2749–2760
Dong HJ, Roeder GS (2000) Organization of the yeast Zip1 protein within the central region of the synaptonemal complex. J Cell Biol 148:417–426
Egel R (1995) The synaptonemal complex and the distribution of meiotic recombination events. Trends Genet 11:206–208
Egel-Mitani M, Olson LW, Egel R (1982) Meiosis in Aspergillus nidulans: another example for lacking synaptonemal complexes in the absence of crossover interference. Hereditas 97:179–187
Fawcett DW (1956) The fine structure of chromosomes in the meiotic prophase of vertebrate spermatocytes. J Biophys Biochem Cytol 2:403–406
Foss E, Lande R, Stahl FW, Steinberg CM (1993) Chiasma interference as a function of genetic distance. Genetics 133:681–691
Froenicke L, Anderson LK, Wienberg J, Ashley T (2002) Male mouse recombination maps for each autosome identified by chromosome painting. Am J Hum Genet 71:1353–1368
Fung JC, Rockmill B, Odell M, Roeder GS (2004) Imposition of crossover interference through the nonrandom distribution of synapsis initiation complexes. Cell 116:795–802
Gerton JL, Hawley RS (2005) Homologous chromosome interactions in meiosis: diversity amidst conservation. Nat Rev Genet 6:477–487
Gong WJ, Mckim KS, Hawley RS (2005) All paired up with no place to go: pairing, synapsis and DSB formation in a balancer heterozygote. PLoS Genet 1:589–602
Gowen MS, Gowen JW (1922) Complete linkage in Drosophila melanogaster. Am Nat 56:286–288
Grelon M, Vezon D, Gendrot G, Pelletier G (2001) AtSPO11-1 is necessary for efficient meiotic recombination in plants. EMBO J 20:589–600
Guillon H, Baudat F, Grey C, Liskay RM, de Massy B (2005) Crossover and noncrossover pathways in mouse meiosis. Mol Cell 20:563–573
Havekes, FWJ (1999) Homologous chromosome pairing and recombination during meiosis in wild type and synaptic mutants of tomato. Thesis, Wageningen University, Wageningen
Henderson KA, Keeney S (2004) Tying synaptonemal complex initiation to the formation and programmed repair of DNA double-strand breaks. Proc Natl Acad Sci U S A 101:4519–4524
Henderson KA, Keeney S (2005) Synaptonemal complex formation: where does it start? Bioessays 27:995–998
Higgins JD, Armstrong SJ, Franklin FCH, Jones GH (2004) The Arabidopsis MutS homolog AtMSH4 functions at an early step in recombination: evidence for two classes of recombination in Arabidopsis. Genes Dev 18:2557–2570
Higgins JD, Sanchez-Moran E, Armstrong SJ, Jones GH, Franklin FC (2005) The Arabidopsis synaptonemal complex protein ZYP1 is required for chromosome synapsis and normal fidelity of crossing over. Genes Dev 19:2488–2500
Hillers KJ (2004) Crossover interference. Curr Biol 14:R1036–R1037
Hillers KJ, Villeneuve AM (2003) Chromosome-wide control of meiotic crossing over in C. elegans. Curr Biol 13:1641–1647
Hodges CA, Revenkova E, Jessberger R, Hassold T, Hunt P (2005) The SMC1b-deficient female mouse: evidence that cohesins are a missing link in age-related nondisjunction. Nat Genet 37:1351–1355
Hollingsworth NM, Brill SJ (2004) The Mus81 solution to resolution: generating meiotic crossovers without Holliday junctions. Genes Dev 18:117–125
Housworth EA, Stahl FW (2003) Crossover interference in humans. Am J Hum Genet 73:188–197
Hunter N (2003) Synaptonemal complexities and commonalities. Mol Cell 12:533–535
Hunter N, Borts RH (1997) Mlh1 is unique among mismatch repair proteins in its ability to promote crossing over during meiosis. Genes Dev 11:1573–1582
Hunter N, Kleckner N (2001) The single-end invasion. An asymmetric intermediate at the double-strand break to double-Holliday junction transition of meiotic recombination. Cell 106:59–70
Jang JK, Sherizen DE, Bhagat R, Manheim EA, McKim KS (2003) Relationship between DNA double-strand breaks to synapsis in Drosophila. J Cell Sci 116:3069–3077
Jazayeri A, Falck J, Lukas C, Bartek J, Smith GCM, Lukas J, Jackson SP (2006) ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol 8:37–45
Jones GH (1987) Chiasmata. In: Moens PB (ed) Meiosis. Academic, Orlando, pp 213–244
Keeney S (2001) Mechanism and control of meiotic recombination initiation. Curr Top Dev Biol 52:1–53
Kleckner N (1996) Meiosis: How could it work? Proc Natl Acad Sci U S A 93:8167–8174
Kleckner N, Zickler D, Jones GH, Dekker J, Padmore R, Henle J, Hutchinson J (2004) A mechanical basis for chromosome function. Proc Natl Acad Sci U S A 101:12592–12597
Klein F, Mahr P, Galova M, Buonomo SB, Michaelis C, Nairz K, Nasmyth K (1999) A central role for cohesins in sister chromatid cohesion, formation of axial elements, and recombination during yeast meiosis. Cell 98:91–103
Koehler KE, Cherry JP, Lynn A, Hunt PA, Hassold TJ (2002) Genetic control of mammalian meiotic recombination. I. Variation in exchange frequencies among males from inbred mouse strains. Genetics 162:297–306
Kohli J, Bähler J (1994) Homologous recombination in fission yeast: absence of crossover interference and synaptonemal complex. Experientia 50:295–306
Lipkin SM, Moens PB, Wang V, Lenzi M, Shanmugarajah D, Gilgeous A, Thomas J, Cheng J, Touchman JW, Green ED, Schwartzberg P, Collins FS, Cohen PE (2002) Meiotic arrest and aneuploidy in MLH3-deficient mice. Nat Genet 31:385–390
Liu J-G, Yuan L, Brundell E, Björkroth B, Daneholt B, Höög C (1996) Localization of the N-terminus of SCP1 to the central element of the synaptonemal complex and evidence for direct interactions between the N-termini of SCP1 molecules organized head-to-head. Exp Cell Res 226:11–19
Loidl J, Scherthan H (2004) Organization and pairing of meiotic chromosomes in the ciliate Tetrahymena thermophila. J Cell Sci 117:5791–5801
Lorenz A, Wells JL, Pryce DW, Novatchkova M, Eisenhaber F, McFarlane RJ, Loidl J (2004) S. pombe meiotic linear elements contain proteins related to synaptonemal complex components. J Cell Sci 117:3343–3351
MacQueen AJ, Colaiácovo MP, McDonald K, Villeneuve AM (2002) Synapsis-dependent and -independent mechanisms stabilize homolog pairing during meiotic prophase in C. elegans. Genes Dev 16:2428–2442
Maguire MP (1980) Adaptive advantage for chiasma interference: a novel suggestion. Heredity 45:127–131
Maguire MP (1992) The evolution of meiosis. J Theor Biol 154:43–55
Mahadevaiah SK, Turner JMA, Baudat F, Rogakou EP, de Boer P, Blanco-Rodriguez J, Jasin M, Keeney S, Bonner WM, Burgoyne PS (2001) Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet 27:271–276
Manheim EA, McKim KS (2003) The synaptonemal complex component C(2)M regulates meiotic crossing over in Drosophila. Curr Biol 13:276–285
Maynard Smith J, Szathmáry E (1995) The major transitions in evolution. Freeman, Oxford
McKee B, Hong C, Das S (2000) On the roles of heterochromatin and euchromatin in meiosis in Drosophila: mapping chromosomal pairing sites and testing candidate mutations for effects on X–Y nondisjunction and meiotic drive in male meiosis. Genetica 109:77–93
McKim KS, Peters K, Rose AM (1993) Two types of sites required for meiotic chromosome pairing in Caenorhabditis elegans. Genetics 134:749–768
McKim KS, Green-Marroquin BL, Sekelsky JJ, Chin G, Steinberg C, Khodosh R, Hawley RS (1998) Meiotic synapsis in the absence of recombination. Science 279:876–878
Mercier R, Jolivet S, Vezon D, Huppe E, Chelysheva L, Giovanni M, Nogue F, Doutriaux MP, Horlow C, Grelon M, Mezard C (2005) Two meiotic crossover classes cohabit in Arabidopsis: one is dependent on MER3, whereas the other one is not. Curr Biol 15:692–701
Meuwissen RLJ, Offenberg HH, Dietrich AJJ, Riesewijk A, van Iersel M, Heyting C (1992) A coiled-coil related protein specific for synapsed regions of meiotic prophase chromosomes. EMBO J 11:5091–5100
Meuwissen RLJ, Meerts I, Hoovers JMN, Leschot NJ, Heyting C (1997) Human synaptonemal complex protein 1 (SCP1): isolation and characterization of the cDNA and chromosomal localization of the gene. Genomics 39:377–384
Moens PB, Tarsounas M, Morita T, Habu T, Rottinghaus ST, Freire R, Jackson SP, Barlow C, Wynshaw BA (1999) The association of ATR protein with mouse meiotic chromosome cores. Chromosoma 108:95–102
Moens PB, Kolas NK, Tarsounas M, Marcon E, Cohen PE, Spyropoulos B (2002) The time course and chromosomal localization of recombination-related proteins at meiosis in the mouse are compatible with models that can resolve the early DNA–DNA interactions without reciprocal recombination. J Cell Sci 115:1611–1622
Molnar M, Bähler J, Sipiczki M, Kohli J (1995) The rec8 gene of Schizosaccharomyces pombe is involved in linear element formation, chromosome pairing and sister-chromatid cohesion during meiosis. Genetics 141:61–73
Moses MJ (1956) Chromosome structures in crayfish spermatocytes. J Biophys Biochem Cytol 2:215–218
Muller HJ (1916) The mechanism of crossing-over. Am Nat 50:193–221
Munz P (1994) Lack of crossover and chromatid interference in Schizosaccharomyces pombe. Genetics 137:701–707
Nasmyth K (2001) Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu Rev Genet 35:673–745
Nasmyth K (2005) How might cohesin hold sister chromatids together? Philos Trans R Soc Lond B Biol Sci 360:483–496
Novak JE, Ross-Macdonald PB, Roeder GS (2001) The budding yeast Msh4 protein functions in chromosome synapsis and the regulation of crossover distribution. Genetics 158:1013–1025
Öllinger R, Alsheimer M, Benavente R (2005) Mammalian protein SCP1 forms synaptonemal complex-like structures in the absence of meiotic chromosomes. Mol Biol Cell 16:212–217
Page SL, Hawley RS (2001) c(3)G encodes a Drosophila synaptonemal complex protein. Genes Dev 15:3130–3143
Page SL, Hawley RS (2004) The genetics and molecular biology of the synaptonemal complex. Annu Rev Cell Dev Biol 20:525–558
Pelttari J, Hoka M-R, Yuan L, Liu J-G, Brundell E, Moens P, Santucci-Darmanin S, Jessberger R, Barbero JL, Heyting C, Höög C (2001) Meiotic chromosomal core consisting of cohesin complex proteins recruits DNA recombination proteins and promotes synapsis in the absence of an axial element in mammalian meiotic cells. Mol Cell Biol 22:5667–5677
Perera D, Perez-Hidalgo L, Moens PB, Reini K, Lakin N, Syväoja JE, San-Segundo PA, Freire R (2004) TopBP1 and ATR co-localization at meiotic chromosomes: role of TopBP1/Cut5 in the meiotic recombination checkpoint. Mol Biol Cell 15:1568–1579
Pochart P, Woltering D, Hollingsworth NM (1997) Conserved properties between functionally distinct MutS homologs in yeast. J Biol Chem 272:30345–30349
Richard GF, Kerrest A, Lafontaine I, Dujon B (2005) Comparative genomics of hemiascomycete yeasts: genes involved in DNA replication, repair and recombination. Mol Biol Evol 22:1011–1023
Rockmill B, Fung JC, Branda SS, Roeder GS (2003) The Sgs1 helicase regulates chromosome synapsis and meiotic crossing over. Curr Biol 13:1954–1962
Roeder GS (1997) Meiotic chromosomes: it takes two to tango. Genes Dev 11:2600–2621
Romanienko PJ, Camerini-Otero RD (2000) The mouse SPO11 gene is required for meiotic chromosome synapsis. Mol Cell 6:975–987
Ross-Macdonald P, Roeder GS (1994) Mutation of a meiosis-specific MutS homolog decreases crossing over but not mismatch correction. Cell 79:1069–1080
Sage J, Martin L, Meuwissen R, Heyting C, Cuzin F, Rassoulzadegan M (1999) Temporal and spatial control of the Sycp1 gene transcription in the mouse meiosis: regulatory elements active in the male are not sufficient for expression in the female gonad. Mech Dev 80:29–39
Santucci-Darmanin S, Walpita D, Lespinasse F, Desnuelle C, Ashley T, Paquis-Flucklinger V (2000) MSH4 acts in conjunction with MLH1 during mammalian meiosis. FASEB J 14:1539–1547
Santucci-Darmanin S, Neyton S, Lespinasse F, Saunieres A, Gaudray P, Paquis-Flucklinger V (2002) The DNA mismatch-repair MLH3 protein interacts with MSH4 in meiotic cells, supporting a role for this MutL homolog in mammalian meiotic recombination. Hum Mol Genet 11:1697–1706
Schleiffer A, Kaitna S, Maurer SS, Glotzer M, Nasmyth K, Eisenhaber F (2003) Kleisins: a superfamily of bacterial and eukaryotic SMC protein partners. Mol Cell 11:571–575
Schmekel K, Meuwissen RLJ, Dietrich AJJ, Vink ACG, van Marle J, van Veen H, Heyting C (1996) Organization of SCP1 protein molecules within synaptonemal complexes of the rat. Exp Cell Res 226:20–30
Schwacha A, Kleckner N (1995) Identification of double Holliday junctions as intermediates in meiotic recombination. Cell 83:783–791
Schwacha A, Kleckner N (1997) Interhomolog bias during meiotic recombination: meiotic functions promote a highly differentiated interhomolog only pathway. Cell 90:1123–1135
Sherizen D, Jang JK, Bhagat R, Kato N, McKim KS (2005) Meiotic recombination in Drosophila females depends on chromosome continuity between genetically defined boundaries. Genetics 169:767–781
Sherman JD, Stack SM (1995) Two-dimensional spreads of synaptonemal complexes from solanaceous plants. IV. High-resolution recombination nodule map for tomato (Lycopersicon esculentum). Genetics 141:683–708
Snowden T, Acharya S, Butz C, Berardini M, Fishel R (2004) hMSH4–hMSH5 recognizes Holliday junctions and forms a meiosis-specific sliding clamp that embraces homologous chromosomes. Mol Cell 15:437–451
Stahl FW, Foss HM, Young LS, Borts RH, Abdullah MFF, Copenhaver GP (2004) Does crossover interference count in Saccharomyces cerevisiae? Genetics 168:35–48
Storlazzi A, Xu L, Schwacha A, Kleckner N (1996) Synaptonemal complex (SC) component Zip1 plays a role in meiotic recombination independent of SC polymerization along the chromosomes. Proc Natl Acad Sci U S A 93:9043–9048
Strickland WN (1958) An analysis of interference in Aspergillus nidulans. Proc Roy Soc B 149:82–101
Sun H, Treco D, Szostak JW (1989) Initiation of meiotic recombination by double-strand DNA-scission. Nature 250:150–153
Suzuki M (1989) SPXX, a frequent sequence motif in gene regulatory proteins. J Mol Biol 207:61–84
Sybenga J (1975) Meiotic configurations. Springer, Berlin Heidelberg New York
Sybenga J (1996) Recombination and chiasmata: few but intriguing discrepancies. Genome 39:473–484
Sym M, Engebrecht JA, Roeder GS (1993) Zip1 is a synaptonemal complex protein required for meiotic chromosome synapsis. Cell 72:365–378
Sym M, Roeder GS (1994) Crossover interference is abolished in the absence of a synaptonemal complex protein. Cell 79:283–292
Sym M, Roeder GS (1994b) Zip1-induced changes in synaptonemal complex structure and polycomplex assembly. J Cell Biol 128:455–466
Szostak JW, Orr-Weaver TL, Rothstein RJ, Stahl FW (1983) The double-strand-break repair model for recombination. Cell 33:25–35
Thompson LH, Schild D (2002) Recombinational DNA repair and human disease. Mutat Res 509:49–78
Tsubouchi T, Roeder GS (2005) A synaptonemal complex protein promotes homology-independent centromere coupling. Science 308:870–873
Tung KS, Roeder GS (1998) Meiotic chromosome morphology and behavior in zip1 mutants of Saccharomyces cerevisiae. Genetics 149:817–832
Turner JMA, Aprelikova O, Xu X, Wang R, Kim S, Chandramouli GVR, Barrett JC, Burgoyne PS, Deng CX (2004) BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr Biol 14:2135–2142
van Heemst D, Heyting C (2000) Sister chromatid cohesion and recombination in meiosis. Chromosoma 109:10–26
van Veen JE, Hawley RS (2003) Meiosis: when even two is a crowd. Curr Biol 13:R831–R833
Wang TF, Kleckner N, Hunter N (1999) Functional specificity of MutL homologs in yeast: evidence for three Mlh1-based heterocomplexes with distinct roles during meiosis in recombination and mismatch correction. Proc Natl Acad Sci U S A 96:13914–13919
Whitby MC (2005) Making crossovers during meiosis. Biochem Soc Trans 33:1451–1455
Woods LM, Hodges CA, Baart E, Baker SM, Liskay M, Hunt PA (1999) Chromosomal influence on meiotic spindle assembly: abnormal meiosis I in female Mlh1 mutant mice. J Cell Biol 145:1395–1406
Wu L, Hickson ID (2003) The Bloom’s syndrome helicase suppresses crossing over during homologous recombination. Nature 426:870–874
Yamamoto A, Hiraoka Y (2001) How do meiotic chromosomes meet their homologous partners?: lessons from fission yeast. Bioessays 23:526–533
Zickler D, Kleckner N (1999) Meiotic chromosomes: integrating structure and function. Annu Rev Genet 33:603–754
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Benavente
The synaptonemal complex—50 years.
Rights and permissions
About this article
Cite this article
de Boer, E., Heyting, C. The diverse roles of transverse filaments of synaptonemal complexes in meiosis. Chromosoma 115, 220–234 (2006). https://doi.org/10.1007/s00412-006-0057-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-006-0057-5