Abstract
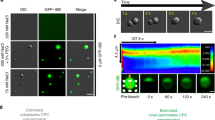
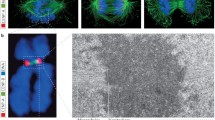
Centromere positioning in human cell nuclei was traced in non-cycling peripheral blood lymphocytes (G0) and in terminally differentiated monocytes, as well as in cycling phytohemagglutinin-stimulated lymphocytes, diploid lymphoblastoid cells, normal fibroblasts, and neuroblastoma SH-EP cells using immunostaining of kinetochores, confocal microscopy and three-dimensional image analysis. Cell cycle stages were identified for each individual cell by a combination of replication labeling with 5-bromo-2′-deoxyuridine and immunostaining of pKi67. We demonstrate that the behavior of centromeres is similar in all cell types studied: a large fraction of centromeres are in the nuclear interior during early G1; in late G1 and early S phase, centromeres shift to the nuclear periphery and fuse in clusters. Peripheral location and clustering of centromeres are most pronounced in non-cycling cells (G0) and terminally differentiated monocytes. In late S and G2, centromeres partially decluster and migrate towards the nuclear interior. In the rather flat nuclei of adherently growing fibroblasts and neuroblastoma cells, kinetochores showed asymmetrical distributions with preferential kinetochore location close either to the bottom side of the nucleus (adjacent to the growth surface) or to the nuclear upper side. This asymmetrical distribution of centromeres is considered to be a consequence of chromosome arrangement in anaphase rosettes.









Similar content being viewed by others
References
Abney JR, Cutler B, Fillbach ML, Axelrod D, Scalettar BA (1997) Chromatin dynamics in interphase nuclei and its implications for nuclear structure. J Cell Biol 137:1459–1468
Abranches R, Beven AF, Aragon-Alcaide L, Shaw PJ (1998) Transcription sites are not correlated with chromosome territories in wheat nuclei. J Cell Biol 143:5–12
Alcobia I, Dilao R, Parreira L (2000) Spatial associations of centromeres in the nuclei of hematopoietic cells: evidence for cell-type-specific organizational patterns. Blood 95:1608–1615
Bartholdi MF (1991) Nuclear distribution of centromeres during the cell cycle of human diploid fibroblasts. J Cell Sci 99:255–263
Baxter J, Merkenschlager M, Fisher AG (2002) Nuclear organisation and gene expression. Curr Opin Cell Biol 14:372–376
Bird A (2002) DNA methylation patterns and epigenetic memory. Genes Dev 16:6–21
Bornfleth H, Edelmann P, Zink D, Cremer T, Cremer C (1999) Quantitative motion analysis of subchromosomal foci in living cells using four-dimensional microscopy. Biophys J 77:2871–2886
Bridger JM, Lichter P (1999) Analysis of mammalian interphase chromosomes by FISH and immunofluorescence. In: Bickmore WA (ed) Chromosome structural analysis. Oxford University, Oxford, pp 103–123
Bridger JM, Kill IR, Lichter P (1998) Association of pKi-67 with satellite DNA of the human genome in early G1 cells. Chromosome Res 6:13–24
Brown KE, Guest SS, Smale ST, Hahm K, Merkenschlager M, Fisher AG (1997) Association of transcriptionally silent genes with Ikaros complexes at centromeric heterochromatin. Cell 91:845–854
Brown KE, Baxter J, Graf D, Merkenschlager M, Fisher AG (1999) Dynamic repositioning of genes in the nucleus of lymphocytes preparing for cell division. Mol Cell 3:207–217
Choo AKH (1997) The centromere. Oxford University Press, Oxford
Cremer M, von Hase J, Volm T, Brero A, Kreth G, Walter J, Fischer C, Solovei I, Cremer C, Cremer T (2001) Non-random radial higher-order chromatin arrangements in nuclei of diploid human cells. Chromosome Res 9:541–567
Csink AK, Henikoff S (1998) Large-scale chromosomal movements during interphase progression in Drosophila. J Cell Biol 143:13–22
van Driel R, Manders EMM, de Jong L, Stap J, Aten JA (1998) Mapping of DNA replication sites in situ by fluorescence microscopy. Methods Cell Biol, pp 455–469
Dundr M, Misteli T (2001) Functional architecture in the cell nucleus. Biochem J 356:297–310
Ferguson M, Ward DC (1992) Cell cycle dependent chromosomal movement in pre-mitotic human T-lymphocyte nuclei. Chromosoma 101:557–565
Gasser SM (2002) Visualizing chromatin dynamics in interphase nuclei. Science 296:1412–1416
Gerlich D, Beaudouin J, Kalbfuss B, Daigle N, Eils R, Ellenberg J (2003) Global chromosome positions are transmitted through mitosis in mammalian cells. Cell 112:751–764
Haaf T, Schmid M (1991) Chromosome topology in mammalian interphase nuclei. Exp Cell Res 192:325–332
Habermann FA, Cremer M, Walter J, Kreth G, von Hase J, Bauer K, Wienberg J, Cremer C, Cremer T, Solovei I (2001) Arrangements of macro- and microchromosomes in chicken cells. Chromosome Res 9:569–584
Hulspas R, Houtsmuller AB, Krijtenburg PJ, Bauman JG, Nanninga N (1994) The nuclear position of pericentromeric DNA of chromosome 11 appears to be random in G0 and non-random in G1 human lymphocytes. Chromosoma 103:286–292
Ikegami S, Taguchi T, Ohashi M, Oguro M, Nagano H, Mano Y (1978) Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature 275:458–460
Kill IR (1996) Localisation of the Ki-67 antigen within the nucleolus. Evidence for a fibrillarin-deficient region of the dense fibrillar component. J Cell Sci 109:1253–1263
Lachner M, Jenuwein T (2002) The many faces of histone lysine methylation. Curr Opin Cell Biol 14:286–298
Lachner M, O’Sullivan RJ, Jenuwein T (2003) An epigenetic road map for histone lysine methylation. J Cell Sci 116:2117–2124
Leonhardt H, Rahn HP, Weinzierl P, Sporbert A, Cremer T, Zink D, Cardoso MC (2000) Dynamics of DNA replication factories in living cells. J Cell Biol 149:271–280
Mahy NL, Perry PE, Bickmore WA (2002) Gene density and transcription influence the localization of chromatin outside of chromosome territories detectable by FISH. J Cell Biol 159:753–763
Maison C, Almouzni G (2004) HP1 and the dynamics of heterochromatin maintenance. Nat Rev Mol Cell Biol 5:296–305
Manders EM, Kimura H, Cook PR (1999) Direct imaging of DNA in living cells reveals the dynamics of chromosome formation. J Cell Biol 144:813–821
Manuelidis L (1984) Different central nervous system cell types display distinct and nonrandom arrangements of satellite DNA sequences. Proc Natl Acad Sci USA 81:3123–3127
Manuelidis L (1985) Indications of centromere movement during interphase and differentiation. Ann N Y Acad Sci 450:205–221
Marshall WF (2002) Order and disorder in the nucleus. Curr Biol 12:R185–R192
Martou G, De Boni U (2000) Nuclear topology of murine, cerebellar Purkinje neurons: changes as a function of development. Exp Cell Res 256:131–139
O’Keefe RT, Henderson SC, Spector DL (1992) Dynamic organization of DNA replication in mammalian cell nuclei: spatially and temporally defined replication of chromosome-specific alpha-satellite DNA sequences. J Cell Biol 116:1095–1110
Parada L, Misteli T (2002) Chromosome positioning in the interphase nucleus. Trends Cell Biol 12:425–432
Rabl C (1885) Über Zelltheilung. In: Gegenbaur C (ed) Morphologisches Jahrbuch, pp 214–330
Richards EJ, Elgin SC (2002) Epigenetic codes for heterochromatin formation and silencing: rounding up the usual suspects. Cell 108:489–500
Solovei I, Walter J, Cremer M, Habermann H, Schermelleh L, Cremer T (2002a) FISH on three-dimensionally preserved nuclei. In: Beatty B, Mai S, Squire J (eds) FISH. Oxford University, Oxford, pp 119–157
Solovei I, Cavallo A, Schermelleh L, Jaunin F, Scasselati C, Cmarko D, Cremer C, Fakan S, Cremer T (2002b) Spatial preservation of nuclear chromatin architecture during three-dimensional fluorescence in situ hybridization (3D-FISH). Exp Cell Res 276:10–23
Solovei I, Grandi N, Knoth R, Volk B, Cremer T (2004a) Postnatal changes of pericentromeric heterochromatin and nucleoli in postmitotic Purkinje cells during murine cerebellum development. Cytogenet Genome Res 105 (in press)
Solovei I, Schermelleh L, Albiez H, Cremer T (2004b) Detection of the cell cycle stages in situ in growing cell populations. In: Celius J (ed) Cell biology: a laboratory handbook, 3rd edn. Elsevier, San Diego (in press)
Starborg M, Gell K, Brundell E, Hoog C (1996) The murine Ki-67 cell proliferation antigen accumulates in the nucleolar and heterochromatic regions of interphase cells and at the periphery of the mitotic chromosomes in a process essential for cell cycle progression. J Cell Sci 109:143–153
Tashiro S, Walter J, Shinohara A, Kamada N, Cremer T (2000) Rad51 accumulation at sites of DNA damage and in postreplicative chromatin. J Cell Biol 150:283–291
Volpi EV, Chevret E, Jones T, Vatcheva R, Williamson J, Beck S, Campbell RD, Goldsworthy M, Powis SH, Ragoussis J, Trowsdale J, Sheer D (2000) Large-scale chromatin organization of the major histocompatibility complex and other regions of human chromosome 6 and its response to interferon in interphase nuclei. J Cell Sci 113:1565–1576
Vourc’h C, Taruscio D, Boyle AL, Ward DC (1993) Cell cycle-dependent distribution of telomeres, centromeres, and chromosome-specific subsatellite domains in the interphase nucleus of mouse lymphocytes. Exp Cell Res 205:142–151
Walter J, Schermelleh L, Cremer M, Tashiro S, Cremer T (2003) Chromosome order in HeLa cells changes during mitosis and early G1, but is stably maintained during subsequent interphase stages. J Cell Biol 160:685–697
Weierich C, Brero A, Stein S, von Hase J, Cremer C, Cremer T, Solovei I (2003) Three-dimensional arrangements of centromeres and telomeres in nuclei of human and murine lymphocytes. Chromosome Res 11:485–502
Weimer R, Haaf T, Kruger J, Poot M, Schmid M (1992) Characterization of centromere arrangements and test for random distribution in G0, G1, S, G2, G1, and early S′ phase in human lymphocytes. Hum Genet 88:673–682
Williams RR, Broad S, Sheer D, Ragoussis J (2002) Subchromosomal positioning of the epidermal differentiation complex (EDC) in keratinocyte and lymphoblast interphase nuclei. Exp Cell Res 272:163–175
Acknowledgements
We are grateful to W.C. Earnshaw (University of Edinburgh) for the generous gift of anti-CENP-B and anti-CENP-C antibodies. B. Joffe (Technical University of Munich) has suggested and greatly helped with the analysis of the asymmetrical distribution of peripheral kinetochores in individual cells. This work was supported by grants from the Deutsche Forschungsgemeinschaft to T. Cremer (Cr 59/20, 1–3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E.A. Nigg
Rights and permissions
About this article
Cite this article
Solovei, I., Schermelleh, L., Düring, K. et al. Differences in centromere positioning of cycling and postmitotic human cell types. Chromosoma 112, 410–423 (2004). https://doi.org/10.1007/s00412-004-0287-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-004-0287-3