Abstract
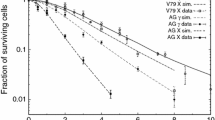
This paper presents a biophysical model of radiation-induced cell death, implemented as a Monte Carlo code called BIophysical ANalysis of Cell death and chromosome Aberrations (BIANCA), based on the assumption that some chromosome aberrations (dicentrics, rings, and large deletions, called “lethal aberrations”) lead to clonogenic inactivation. In turn, chromosome aberrations are assumed to derive from clustered, and thus severe, DNA lesions (called “cluster lesions,” or CL) interacting at the micrometer scale; the CL yield and the threshold distance governing CL interaction are the only model parameters. After a pilot study on V79 hamster cells exposed to protons and carbon ions, in the present work the model was extended and applied to AG1522 human cells exposed to photons, He ions, and heavier ions including carbon and neon. The agreement with experimental survival data taken from the literature supported the assumptions. In particular, the inactivation of AG1522 cells was explained by lethal aberrations not only for X-rays, as already reported by others, but also for the aforementioned radiation types. Furthermore, the results are consistent with the hypothesis that the critical initial lesions leading to cell death are DNA cluster lesions having yields in the order of ~2 CL Gy−1 cell−1 at low LET and ~20 CL Gy−1 cell−1 at high LET, and that the processing of these lesions is modulated by proximity effects at the micrometer scale related to interphase chromatin organization. The model was then applied to calculate the fraction of inactivated cells, as well as the yields of lethal aberrations and cluster lesions, as a function of LET; the results showed a maximum around 130 keV/μm, and such maximum was much higher for cluster lesions and lethal aberrations than for cell inactivation.





Similar content being viewed by others
References
Ballarini F (2010) From DNA radiation damage to cell death: theoretical approaches. J Nucleic Acids 2010, Article ID 350608. doi:10.4061/2010/350608
Ballarini F, Ottolenghi A (2003) Chromosome aberrations as biomarkers of radiation exposure: modelling basic mechanisms. Adv Space Res 31:1557–1568
Ballarini F, Ottolenghi A (2004) A model of chromosome aberration induction and CML incidence at low doses. Radiat Environ Biophys 43:165–171
Ballarini F, Ottolenghi A (2005) A model of chromosome aberration induction: applications to space research. Radiat Res 164:567–570
Ballarini F, Merzagora M, Monforti F, Durante M, Gialanella G, Grossi GF et al (1999) Chromosome aberrations induced by light ions: Monte carlo simulations based on a mechanistic model. Int J Radiat Biol 75:35–46
Ballarini F, Alloni D, Facoetti A, Ottolenghi A (2008) Heavy-ion effects: from track structure to DNA and chromosome damage. New J Phys 10:075008. http://www.njp.org
Ballarini F, Bortolussi S, Clerici AM, Ferrari C, Protti N, Altieri S (2011) From radiation-induced chromosome damage to cell death: modelling basic mechanisms and applications to boron neutron capture therapy. Radiat Prot Dosim 143(2–4):523–527
Ballarini F, Altieri S, Bortolussi S, Giroletti E, Protti N (2013) A model of radiation-induced cell killing: insights into mechanisms and applications for hadron therapy. Radiat Res 180:307–315
Brenner DJ, Miller RC, Huang Y, Hall EJ (1995) The biological effectiveness of radon-progeny alpha particles III. Quality factors. Radiat Res 142:61–69
Campa A, Ballarini F, Belli M, Cherubini R, Dini V, Esposito G, Friedland W, Gerardi S, Molinelli S, Ottolenghi A, Paretzke HG, Simone G, Tabocchini MA (2005) DNA DSB induced in human cells by charged particles and gamma rays: experimental results and theoretical approaches. Int J Radiat Biol 81:841–854
Campa A, Alloni D, Antonelli F, Ballarini F, Belli M, Dini V, Esposito G, Facoetti A, Friedland W, Furusawa Y, Liotta M, Ottolenghi A, Paretzke HG, Simone G, Sorrentino E, Tabocchini MA (2009) DNA fragmentation induced in human fibroblasts by 56Fe ions: experimental data and MC simulations. Radiat Res 171:438–445
Chadwick K, Leenhouts H (1973) A molecular theory of cell survival. Phys Med Biol 18:78–87
Chatterjee A, Schaefer HJ (1976) Microdosimetric structure of heavy ion tracks in tissue. Radiat Environ Biophys 13:215–227
Cornforth M, Bedford J (1987) A quantitative comparison of potentially lethal damage repair and the rejoining of interphase chromosome breaks in low passage normal human fibroblasts. Radiat Res 111:385–405
Cornforth MN, Bailey SM, Goodwin EH (2002) Dose responses for chromosome aberrations produced in noncycling primary human fibroblasts by alpha particles, and by gamma rays delivered at sublimiting low dose rates. Radiat Res 158:43–53
Cucinotta FA, Nikjoo H, Goodhead DT (1999) Applications of amorphous track models in radiation biology. Radiat Environ Biophys 38:81–92
Curtis SB (1986) Lethal and potentially lethal lesions induced by radiation—a unified repair model. Radiat Res 106:252–271
Durante M, Loeffler JS (2010) Charged particles in radiation oncology. Nat Rev Clin Oncol 7:37–43
Durante M, Bedford JS, Chen DJ, Conrad S, Cornforth MN, Natarajan AT, van Gent DC, Obe G (2013) From DNA damage to chromosome aberrations: joining the break. Mutat Res 756(1–2):5–13
Elsasser T, Kramer M, Scholz M (2008) Accuracy of the local effect model for the prediction of biologic effects of carbon ion beams in vitro and in vivo. Int J Radiat Oncol Biol Phys 71:866–872
Friedland W, Kundrat P (2013) Track structure based modelling of chromosome aberrations after photon and alpha-particle irradiation. Mutat Res 756(1–2):213–223
Friedrich T, Durante M, Scholz M (2012) Modelling cell survival after photon irradiation based on double-strand break clustering in megabase pair chromatin loops. Radiat Res 178:385–394
Georgakilas AG, O’Neill P, Stewart RD (2013) Induction and repair of clustered DNA lesions: what do we know so far? Radiat Res 180(1):100–109
Goodhead DT (1994) Initial events in the cellular effects of ionizing radiations: clustered damage in DNA. Int J Radiat Biol 65:6–17
Hamada N, Funayama T, Wada S, Sakashita T, Kakizaki T, Ni M, Kobayashi Y (2006) LET-dependent survival of irradiated normal human fibroblasts and their descendents. Radiat Res 166:24–30
Kellerer AM, Rossi HH (1972) The theory of dual radiation action. Curr Top Radiat Res Q 8:85–158
Lea DE (1955) Actions of radiations on living cells, 2nd edn. Cambridge University Press, New York
Neti P, de Toledo SM, Perumal V, Azzam EI, Howell RW (2004) A multi-port low-fluence alpha-particle irradiator: fabrication, testing and benchmark radiobiology studies. Radiat Res 161:732–738
Neumaier T, Swenson J, Pham C, Polyzos A, Lo AT, Yang P-A, Dyball J, Asaithamby A, Chen DJ, Thalhammer S, Bissell MJ, Costes SV (2012) Evidence for formation of DNA repair centres and dose response nonlinearity in human cells. PNAS 109:443–448
Newhauser WD, Durante M (2011) Assessing the risk of second malignancies after modern radiotherapy. Nature 11:438–448
Nikjoo H, O’Neill P, Terrissol M, Goodhead DT (1999) Quantitative modelling of DNA damage using Monte Carlo track structure method. Radiat Environ Biophys 38:31–38
Ottolenghi A, Merzagora M, Tallone L, Durante M, Paretzke HG, Wilson WE et al (1995) The quality of DNA double-strand breaks: a Monte Carlo simulation of the end-structure of strand breaks produced by protons and alpha particles. Radiat Environ Biophys 34:239–244
Ottolenghi A, Ballarini F, Biaggi M (2001) Modelling chromosomal aberration induction by ionising radiation: the influence of interphase chromosome architecture. Adv Space Res 27:369–382
Plante I, Ponomarev AL, Cucinotta FA (2013) Calculation of the energy deposition in nano volumes by protons and HZE particles: geometric patterns of initial distributions of DNA repair foci. Phys Med Biol 58(18):6393–6405
Ponomarev AL, George K, Cucinotta FA (2012) Computational model of chromosome aberration yield induced by high- and low-LET radiation exposures. Radiat Res 177:727–737
Sachs RK, Brenner DJ, Hahnfeldt PJ, Hlatky LR (1998) A formalism for analyzing large-scale clustering of radiation-induced breaks along chromosomes. Int J Radiat Biol 74:185–206
Savage JRK (2000) Cancer. Proximity matters. Science 290:62–63
Schipler A, Iliakis G (2013) DNA double-strand break complexity levels and their possible contributions to the probability for error-prone processing and repair pathway choice. Nucleic Acids Res 41(16):7589–7605
Taleei R, Girard PM, Sankaranarayanan K, Nikjoo H (2013) The non-homologous end-joining (NHEJ) mathematical model for the repair of double-strand breaks: II. Application to damage induced by ultrasoft X-rays and low-energy electrons. Radiat Res 179(5):540–548
Tobias CA, Blakely EA, Ngo FQH, Yang TCH (1980) The repair-misrepair model of cell survival. In: Meyn RE, Withers HR (eds) Radiation biology and cancer research. Raven Press, New York, pp 195–230
Zirbel R, Mathieu UR, Kurz A, Cremer T, Lichter P (1993) Evidence for a nuclear compartment of transcription and splicing located at chromosome domain boundaries. Chromosome Res 1:93–106
Acknowledgments
The authors are grateful for useful discussions to Michael Cornforth and Marco Durante. This work was supported by Istituto Nazionale di Fisica Nucleare (project “MiMo-Bragg”).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ballarini, F., Altieri, S., Bortolussi, S. et al. The BIANCA model/code of radiation-induced cell death: application to human cells exposed to different radiation types. Radiat Environ Biophys 53, 525–533 (2014). https://doi.org/10.1007/s00411-014-0537-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-014-0537-6