Abstract
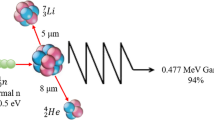
We have shown that boron neutron capture therapy (BNCT) could be an alternative for the treatment of poorly differentiated thyroid carcinoma (PDTC). Histone deacetylase inhibitors (HDACI) like sodium butyrate (NaB) cause hyperacetylation of histone proteins and show capacity to increase the gamma irradiation effect. The purpose of these studies was to investigate the use of the NaB as a radiosensitizer of the BNCT for PDTC. Follicular thyroid carcinoma cells (WRO) and rat thyroid epithelial cells (FRTL-5) were incubated with 1 mM NaB and then treated with boronophenylalanine 10BPA (10 μg 10B ml−1) + neutrons, or with 2, 4-bis (α,β-dihydroxyethyl)-deutero-porphyrin IX 10BOPP (10 μg10B ml−1) + neutrons, or with a neutron beam alone. The cells were irradiated in the thermal column facility of the RA-3 reactor (flux = (1.0 ± 0.1) × 1010 n cm−2 s−1). Cell survival decreased as a function of the physical absorbed dose in both cell lines. Moreover, the addition of NaB decreased cell survival (p < 0.05) in WRO cells incubated with both boron compounds. NaB increased the percentage of necrotic and apoptotic cells in both BNCT groups (p < 0.05). An accumulation of cells in G2/M phase at 24 h was observed for all the irradiated groups and the addition of NaB increased this percentage. Biodistribution studies of BPA (350 mg kg−1 body weight) 24 h after NaB injection were performed. The in vivo studies showed that NaB treatment increases the amount of boron in the tumor at 2-h post-BPA injection (p < 0.01). We conclude that NaB could be used as a radiosensitizer for the treatment of thyroid carcinoma by BNCT.





Similar content being viewed by others
References
Arundel CM, Glicksman AS, Leith JT (1985) Enhancement of radiation injury in human colon tumor cells by the maturational agent sodium butyrate (NaB). Radiat Res 104:443–448
Brierley JD, Tsang RW (2008) External beam radiation therapy for thyroid cancer. Endocrinol Metab Clin North Am 37:497–509
Butler LM, Webb Y, Agus DB, Higgins B, Tolentino TR, Kutko MC, LaQuaglia MP, Drobnjak M, Cordon-Cardo C, Scher HI, Breslow R, Richon VM, Rifkind RA, Marks PA (2001) Inhibition of transformed cell growth and induction of cellular differentiation by pyroxamide, an inhibitor of histone deacetylase. Clin Cancer Res 7:962–970
Camphausen K, Cerna D, Scott T, Sproull M, Burgan WE, Cerra MA, Fine H, Tofilon PJ (2005) Enhancement of in vitro and in vivo tumor cell radiosensitivity by valproic acid. Int J Cancer 114:380–386
Cho HJ, Kim SY, Kim KH, Kang WK, Kim JI, Oh ST, Kim JS, An CH (2009) The combination effect of sodium butyrate and 5-Aza-2′-deoxycytidine on radiosensitivity in RKO colorectal cancer and MCF-7 breast cancer cell lines. World J Surg Oncol 7:49. doi:10.1186/1477-7819-7-49
Dagrosa MA, Thomasz L, Longhino J, Perona M, Calzetta O, Blaumann H, Jimenez Rebagliati R, Cabrini R, Kahl S, Juvenal GJ, Pisarev M (2007) Optimization of boron neutron capture therapy for the treatment of undifferentiated thyroid cancer. Int J Radiat Oncology Biol Phys 69:1059–1066
Detta A, Cruickshank GS (2009) l-amino acid transporter-1 and boronophenylalanine-based boron neutron capture therapy of human brain tumors. Cancer Res 69:2126–2132
Dokmanovic M, Clarke K, Marks P (2007) Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res 5:981–989
Joensuu H, Kankaanranta L, Tenhunen M, Saarilahti K (2011) Boron neutron capture therapy (BNCT) as cancer treatment. Duodecim 127:1697–1703
Kageji T, Mizobuchi Y, Nagahiro S, Nakagawa Y, Kumada H (2011) Clinical results of boron neutron capture therapy (BNCT) for glioblastoma. Appl Radiat Isot 69:1823–1825
Kahl SB, Koo MS (1992) Synthesis and properties of tetrakiscarborane-carboxylate esters of 2, 4-bis (-dihydroxyethyl) deuteroporphyrin IX. In: Allen BJ, Moore DE, Harrington BV (eds) Progress in Neutron Capture Therapy for Cancer. New York Plenum Press, pp 223–226
Koprinarova M, Markovska P, Iliev I, Anachkova B, Russev G (2010) Sodium butyrate enhances the cytotoxic effect of cisplatin by abrogating the cisplatin imposed cell cycle arrest. BMC Mol Biol 11:49
Lee JH, Choy ML, Ngo L, Foster SS, Marks PA (2010) Histone deacetylase inhibitor induces DNA damage which normal but not transformed cells can repair. PNAS 107:14639–14644
Leith JT (1988) Effects of sodium butyrate and 3-aminobenzamide on survival of Chinese hamster HA-1 cells after X irradiation. Radiat Res 114:186–191
Maggio SC, Rosato RR, Kramer LB, Dai Y, Rahmani M, Paik DS, Czarnik AC, Payne SG, Spiegel S, Grant S (2004) The histone deacetylase inhibitor MS-275 interacts synergistically with fludarabine to induce apoptosis in human leukemia cells. Cancer Res 64:2590–2600
Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK (2001) Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 3:194–202
Miller ME, Quintana J, Ojeda J, Langan S, Thorp SI, Pozzi E, Sztejnberg ML, Estryk G, Nosal R, Saire E, Agrazar H, Graiño F (2009) New irradiation facility for biomedical applications at the RA-3 reactor thermal column treatments. Appl Radiat Isot 67:S226–S229
Muhlethaler-Mottet A, Meier R, Flahaut M, Bourloud KB, Nardou K, Joseph JM, Gross N (2008) Complex molecular mechanisms cooperate to mediate histone deacetylase inhibitors anti-tumor activity in neuroblastoma cells. BMC Mol Cancer 7:55
Munshi A, Kurland JF, Nishikawa T, Tanaka T, Hobbs ML, Tucker SL, Ismail S, Stevens C, Meyn RE (2005) Histone deacetylase inhibitors radiosensitize human melanoma cells by suppressing DNA repair activity. Clin Cancer Res 11:4912–4922
Pelicci PG, Minucci S (2006) Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 6:38–51
Pilch DR, Sedelnikova OA, Redon C, Celeste A, Nussenzweig A, Bonner WM (2003) Characteristics of gamma-H2AX foci at DNA double-strand breaks sites. Biochem Cell Biol 81:123–129
Pisarev MA, Dagrosa MA, Juvenal GJ (2007) Boron neutron capture therapy in cancer: past, present and future. Arq Bras Endocrinol Metabol 51:852–856
Puppin C, D’Aurizio F, D’Elia AV, Cesaratto L, Tell G, Russo D, Filetti S, Ferretti E, Tosi E, Mattei T, Pianta A, Pellizzari L, Damante G (2005) Effects of histone acetylation on sodium iodide symporter promoter and expression of thyroid-specific transcription factors. Endocrinology 146:3967–3974
Qiu L, Burgess A, Fairlie DP, Leonard H, Parsons PG, Gabrielli BG (2000) Histone deacetylase inhibitors trigger a G2 checkpoint in normal cells that is defective in tumor cells. Mol Biol Cell 11:2069–2083
Richon VM, Sandhoff TW, Rifkind RA, Marks PA (2000) Histone deacetylase inhibitor induces p21 expression and gene associated histone acetylation. Proc Natl Acad Sci USA 97:10013–10019
Ruefli AA, Ausserlechner MJ, Bernhard D, Sutton VR, Tainton KM, Kofler R, Smyth MJ, Johnstone RW (2005) The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces cell death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc Natl Acad Sci USA 98:10833–10838
Ungerstedt JS, Sowa Y, Xu WS, Shao Y, Dokmanovic M, Perez G, Ngo L, Holmgren A, Jiang X, Marks PA (2005) Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc Natl Acad Sci USA 102:673–678
US Department of Health and Human Services (1985) Guide for the care and use of laboratories animals. Public Health Service, National Institute of Health NIH Publication 86–23
Vrana JA, Decker RH, Johnson CR, Wang Z, Jarvis WD, Richon VM, Ehinger M, Fisher PB, Grant S (1999) Induction of apoptosis in U937 human leukemia cells by suberoylanilide hydroxamic acid (SAHA) proceeds through pathways that are regulated by Bcl-2/Bcl-x (L), c-Jun, and p21 (CIP1), but independent of p53. Oncogene 18:7016–7025
Westphal EM, Blackstock W, Feng W, Israel B, Kenney SC (2000) Activation of lytic Epstein-Barr virus (EBV) infection by radiation and sodium butyrate in vitro and in vivo: a potential method for treating EBV-positive malignancies. Cancer Res 60:5781–5788
Xu WS, Parmigiani RB, Marks PA (2007) Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene 26:5541–5552
Zhang Y, Jung M, Dritschilo A, Jung M (2004) Enhancement of radiation sensitivity of human squamous carcinoma cells by histone deacetylase inhibitors. Radiat Res 161:667–674
Zhang Y, Bao YL, Wu Y, Yu CL, Sun Y, Li YX (2010) Identification and characterization of the human SLC5A8 gene promoter. Cancer Genet Cytogenet 196:124–132
Acknowledgments
This work was supported by grants from the Balseiro Foundation, the National Scientific and Technical Research Council (CONICET) and the National Agency of Scientific and Technological Promotion (ANPCYT).
Conflict of interest
The authors declare that there is no conflict of interest in this paper.
Author information
Authors and Affiliations
Corresponding authors
Additional information
A. Dagrosa and G. Juvenal should be considered as joint authors.
Rights and permissions
About this article
Cite this article
Perona, M., Rodríguez, C., Carpano, M. et al. Improvement of the boron neutron capture therapy (BNCT) by the previous administration of the histone deacetylase inhibitor sodium butyrate for the treatment of thyroid carcinoma. Radiat Environ Biophys 52, 363–373 (2013). https://doi.org/10.1007/s00411-013-0470-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-013-0470-0