Abstract
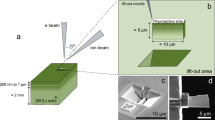
Chemical interdiffusion of Fe–Mg along the c-axis [001] in natural diopside crystals (X Di = 0.93) was experimentally studied at ambient pressure, at temperatures ranging from 800 to 1,200 °C and oxygen fugacities from 10−11 to 10−17 bar. Diffusion couples were prepared by ablating an olivine (X Fo = 0.3) target to deposit a thin film (20–100 nm) onto a polished surface of a natural, oriented diopside crystal using the pulsed laser deposition technique. After diffusion anneals, compositional depth profiles at the near surface region (~400 nm) were measured using Rutherford backscattering spectroscopy. In the experimental temperature and compositional range, no strong dependence of D Fe–Mg on composition of clinopyroxene (Fe/Mg ratio between Di93–Di65) or oxygen fugacity could be detected within the resolution of the study. The lack of fO2-dependence may be related to the relatively high Al content of the crystals used in this study. Diffusion coefficients, D Fe–Mg, can be described by a single Arrhenius relation with
D Fe–Mg in clinopyroxene appears to be faster than diffusion involving Ca-species (e.g., D Ca–Mg) while it is slower than D Fe–Mg in other common mafic minerals (spinel, olivine, garnet, and orthopyroxene). As a consequence, diffusion in clinopyroxene may be the rate-limiting process for the freezing of many geothermometers, and compositional zoning in clinopyroxene may preserve records of a higher (compared to that preserved in other coexisting mafic minerals) temperature segment of the thermal history of a rock. In the absence of pervasive recrystallization, clinopyroxene grains will retain compositions from peak temperatures at their cores in most geological and planetary settings where peak temperatures did not exceed ~1,100 °C (e.g., resetting may be expected in slowly cooled mantle rocks, many plutonic mafic rocks, or ultra-high temperature metamorphic rocks).










Similar content being viewed by others
References
Borinski SA, Hoppe U, Chakraborty S, Ganguly J, Bhowmik SK (2012) Multicomponent diffusion in garnets I: general theoretical considerations and experimental data for Fe-Mg systems. Contrib Miner Petrol 164(4):571–586. doi:10.1007/s00410-012-0758-0
Brady JB, McCallister RH (1983) Diffusion data for clinopyroxenes from homogenization and self-diffusion experiments. Am Mineral 68(1–2):95–105
Cherniak DJ, Dimanov A (2010) Diffusion in pyroxene, mica and amphibole. Rev Mineral Geochem 72(1):641–690. doi:10.2138/rmg.2010.72.14
Dimanov A, Sautter V (2000) “Average” interdiffusion of (Fe, Mn)-Mg in natural diopside. Eur J Mineral 12(4):749–760
Dimanov A, Wiedenbeck M (2006) (Fe, Mn)-Mg interdiffusion in natural diopside: effect of pO(2). Eur J Mineral 18(6):705–718. doi:10.1127/0935-1221/2006/0018-0705
Dimanov A, Jaoul O, Sautter V (1996) Calcium self-diffusion in natural diopside single crystals. Geochim Cosmochim Acta 60(21):4095–4106. doi:10.1016/s0016-7037(96)00250-5
Dodson MH (1973) Closure temperature in cooling geochronological and petrological systems. Contrib Miner Petrol 40(3):259–274
Dodson MH (1986) Closure profiles in cooling systems. In: Freer R, Dennis PF (eds) Kinetics & mass transport in silicate and oxide systems, Materials science forum, vol 7. Trans Tech Publications, Aedermannsdorf, Switzerland, pp 145–154
Dohmen R (2008) A new experimental thin film approach to study mobility and partitioning of elements in grain boundaries: Fe-Mg exchange between olivines mediated by transport through an inert grain boundary. Am Mineral 93(5–6):863–874. doi:10.2138/am 2008.2671
Dohmen R, Chakraborty S (2003) Mechanism and kinetics of element and isotopic exchange mediated by a fluid phase. Am Mineral 88(8–9):1251–1270
Dohmen R, Chakraborty S (2007) Fe-Mg diffusion in olivine II: point defect chemistry, change of diffusion mechanisms and a model for calculation of diffusion coefficients in natural olivine (vol 34, pg 409, 2007). Phys Chem Miner 34:597–598. doi:10.1007/s00269-007-0185-3
Dohmen R, Becker HW, Meissner E, Etzel T, Chakraborty S (2002) Production of silicate thin films using pulsed laser deposition (PLD) and applications to studies in mineral kinetics. Eur J Mineral 14(6):1155–1168. doi:10.1127/0935-1221/2002/0014-1155
Dohmen R, Becker H-W, Chakraborty S (2007) Fe–Mg diffusion in olivine I: experimental determination between 700 and 1,200 °C as a function of composition, crystal orientation and oxygen fugacity. Phys Chem Miner 34(6):389–407
Eiler JM, Baumgartner LP, Valley JW (1994) Fast grain-boundary—a FORTRAN-77 program for calculating the effects of retrograde interdiffusion of stable isotopes. Comput Geosci 20(10):1415–1434. doi:10.1016/0098-3004(94)90102-3
Feldmann LC (1986) Fundamentals of surface and thin film analysis. North-Holland, New York
Frost BR, Chacko T (1989) The granulite uncertainty principle: limitations on thermobarometry in granulites. J Geol 97(4):435–450
Fujino K, Nachara H, Momoi H (1990) Direct determination of cation diffusion coefficients in pyroxenes. Eos 71:943–944
Ganguly J (1979) Garnet and clinopyroxene solid solutions, and geothermometry based on Fe-Mg distribution coefficient. Geochim Cosmochim Acta 43(7):1021–1029
Ganguly J, Tirone M (1999) Diffusion closure temperature and age of a mineral with arbitrary extent of diffusion: theoretical formulation and applications. Earth Planet Sci Lett 170(1–2):131–140
Ganguly J, Bhattacharya RN, Chakraborty S (1988) Convolution effect in the determination of composition profiles and diffusion coefficients by microprobe step scans. Am Mineral 73(7–8):901–909
Ganguly J, Cheng WJ, Tirone M (1996) Thermodynamics of aluminosilicate garnet solid solution: new experimental data, an optimized model, and thermometric applications. Contrib Miner Petrol 126(1–2):137–151. doi:10.1007/s004100050240
Ganguly J, Tirone M, Chakraborty S, Domanik K (2013) H-chondrite parent asteroid: a multistage cooling, fragmentation and re-accretion history constrained by thermometric studies, diffusion kinetic modeling and geochronological data. Geochim Cosmochim Acta 105(0):206–220. doi:10.1016/j.gca.2012.11.024
Green T, Adam J (1991) Assessment of the garnet–clinopyroxene Fe–Mg exchange thermometer using new experimental data. J Metamorph Geol 9(3):341–347
Jaoul O, Raterron P (1994) High-temperature deformation of diopside crystal. 3. Influences of pO2 and SiO2 precipitation. J Geophys Res-Solid Earth 99:9423–9439. doi:10.1029/93jb03363
Kótai E (1994) Computer methods for analysis and simulation of RBS and ERDA spectra. Nucl Instrum Methods Phys Res, Sect B 85(1):588–596
Krogh Ravna E, Terry MP (2004) Geothermobarometry of UHP and HP eclogites and schists–an evaluation of equilibria among garnet–clinopyroxene–kyanite–phengite–coesite/quartz. J Metamorph Geol 22(6):579–592
Liermann HP, Ganguly J (2002) Diffusion kinetics of Fe2+ and Mg in aluminous spinel: experimental determination and applications. Geochim Cosmochim Acta 66(16):2903–2913. doi:10.1016/s0016-7037(02)00875-x
Loucks RR (1996) A precise olivine-augite Mg-Fe-exchange geothermometer. Contrib Miner Petrol 125(2–3):140–150
Lovering TS (1936) Heat conduction in dissimilar rocks and the use of thermal models. Bull Geol Soc Am 47:87–100
Mueller T, Watson EB, Harrison TM (2010) Applications of diffusion data to high-temperature earth systems. Rev Mineral Geochem 72(1):997–1038. doi:10.2138/rmg.2010.72.23
Onorato P, Hopper R, Yinnon H, Uhlmann D, Taylor L, Garrison J, Hunter R (1981) Solute partitioning under continuous cooling conditions as a cooling rate indicator. J Geophys Res 86(10):9511–9518
Pattison D, Newton R (1989) Reversed experimental calibration of the garnet-clinopyroxene Fe–Mg exchange thermometer. Contrib Miner Petrol 101(1):87–103
Pattison DRM, Chako T, Farquhar J, McFarlane CRM (2003) Temperatures of granulite-facies metamorphism: constraints from experimental phase equilibria and thermobarometry corrected for retrograde exchange. J Petrol 44(5):867–900
Perkins D, Vielzeuf D (1992) Experimental investigation of Fe–Mg distribution between olivine and clinopyroxene: implications for mixing properties of Fe-Mg in clinopyroxene and garnet-clinopyroxene thermometry. Am Mineral 77:774–783
Putirka KD, Mikaelian H, Ryerson F, Shaw H (2003) New clinopyroxene-liquid thermobarometers for mafic, evolved, and volatile-bearing lava compositions, with applications to lavas from Tibet and the Snake River Plain, Idaho. American Mineral 88(10):1542–1554
Råheim A, Green DH (1974) Experimental determination of the temperature and pressure dependence of the Fe-Mg partition coefficient for coexisting garnet and clinopyroxene. Contrib Miner Petrol 48(3):179–203
Ravna K (2002) The garnet–clinopyroxene Fe2+–Mg geothermometer: an updated calibration. J Metamorph Geol 18(2):211–219
Rietmeijer FJ (1983) Inter-diffusion coefficients parallel to the c-axis in iron-rich clinopyroxenes calculated from microstructures. Contrib Miner Petrol 83(1–2):169–176
Rietmeijer FJM, Champness PE (1982) Exsolution structures in calcic pyroxenes from the Bjerkreim-Skondal lopolith, SW Norway. Mineral Mag 45:11–24. doi:10.1180/minmag.1982.045.337.02
Smith D, Wilson CR (1985) Garnet-olivine equilibration during cooling in the mantle. Am Mineral 70(1–2):30–39
Watson EB, Dohmen R (2010) Non-traditional and emerging methods for characterizing diffusion in minerals and mineral aggregates. Rev Mineral Geochem 72(1):61–105
Wells PR (1977) Pyroxene thermometry in simple and complex systems. Contrib Miner Petrol 62(2):129–139
Wood BJ, Banno S (1973) Garnet-orthopyroxene and orthopyroxene-clinopyroxene relationships in simple and complex systems. Contrib Miner Petrol 42(2):109–124
Zack T, Foley SF, Jenner GA (1997) A consistent partition coefficient set for clinopyroxene, amphibole and garnet from laser ablation microprobe analysis of garnet pyroxenites from Kakanui, New Zealand. Neues Jahrbuch Fur Mineralogie-Abhandlungen 172(1):23–42
Zhang XY, Ganguly J, Ito M (2010) Ca-Mg diffusion in diopside: tracer and chemical inter-diffusion coefficients. Contrib Miner Petrol 159(2):175–186. doi:10.1007/s00410-009-0422-5
Acknowledgments
This studied was financially supported by grants from the DFG and the Ruhr-Universität Bochum to S. Chakraborty that funded the positions of T.M., R.D. and J.T.H. as well as the running of the experimental petrology and the thin film deposition labs. We gratefully acknowledge the constructive comments of Daniele Cherniak, an anonymous reviewer, and the handling editor Jon Blundy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Blundy.
Rights and permissions
About this article
Cite this article
Müller, T., Dohmen, R., Becker, H.W. et al. Fe–Mg interdiffusion rates in clinopyroxene: experimental data and implications for Fe–Mg exchange geothermometers. Contrib Mineral Petrol 166, 1563–1576 (2013). https://doi.org/10.1007/s00410-013-0941-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00410-013-0941-y