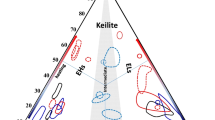
Abstract
Djerfisherite is an important carrier of potassium in highly reduced enstatite chondrites, where it occurs in sub-round metal-sulfide nodules. These nodules were once free-floating objects in the protoplanetary nebula. Here, we analyze existing and new data to derive an equation of state (EOS) for djerfisherites of \( {\text{K}}_{ 6} ({\text{Cu}},{\text{Fe}},{\text{Ni}})^{B} ({\text{Fe}},{\text{Ni}},{\text{Cu}})_{24}^{C} {\text{S}}_{ 2 6} {\text{Cl}} \) structural formula. We use this EOS to calculate the thermal stability of djerfisherite coexisting in equilibrium with a cooling vapor of solar composition enriched in a dust analogous to anhydrous, chondritic interplanetary dust (C-IDP). We find that condensed mineral assemblages closely match those found in enstatite chondrites, with djerfisherite condensing above 1,000 K in C-IDP dust-enriched systems. Results may have implications for the volatile budgets of terrestrial planets and the incorporation of K into early formed, highly reduced, planetary cores. Previous work links enstatite chondrites to the planet Mercury, where the surface has a terrestrial K/Th ratio, high S/Si ratio, and very low FeO content. Mercury’s accretion history may yield insights into Earth’s.





Similar content being viewed by others
References
Anderson GM, Crerar D (1993) Thermodynamics in geochemistry. Oxford University Press, New York
Balabin AI, Sack RO (2000) Thermodynamics of (Zn, Fe) sphalerite: a CVM approach based on large basis clusters. Mineral Mag 64:943–963
Balabin AI, Urosov VS (1995) Recalibration of the sphalerite cosmobarometer: experimental and theoretical treatment. Geochim Cosmochim Acta 59:1401–1410
Barkovi AY, Laajoki KVO, Gehör SA, Yakovlev YN, Taikina-Aho O (1997) Chlorine-poor analogues of djerfisherite: thalfenisite from Noril′sk, Siberia and Salmagorsky, Kola Peninsula, Russia. Can Mineral 35:1421–1430
Benz W, Slattery WL, Cameron AGW (1988) Collisional stripping of Mercury’s mantle. Icarus 74:516–528
Benz W, Anic A, Horner J, Whitby JA (2007) The origin of Mercury. Space Sci Rev 132:189–202
Burbine TH, McCoy TJ, Nittler LR, Benedix GK, Cloutis EA, Dickinson TL (2002) Spectra of extremely reduced assemblages: implications for mercury. Meteor Planet Sci 37:1233–1244
Chabot NL, Drake MJ (1999) Crystallization of magmatic iron meteorites: the role of mixing in the molten core. Meteor Planet Sci 34:235–246
Chase MW Jr (1998) NIST-JANAF thermochemical tables, vol 9, 4th edn, Journal of Physical and Chemical Reference Data, National Institute of Standards and Technology, Washington DC
Clarke DB (1979) Synthesis of nickeloan djerfisherites and the origin of potassic sulfides at the Frank Smith Mine. In Boyd FR, Meyer HOA (eds) The mantle sample: inclusion in kimberlites and other volcanics, proceedings of second international Kimberlite conference, Am Geophys Union, Washington DC, pp 300–330
Clarke DB, Mitchell RH, Chapman CAT, MacKay RM (1994) Occurrence and origin of djerfisherite from the Elwin Bay kimberlite, Somerset Island, Northwest Territories. Can Mineral 32:815–823
Clay PL, King A, Wieler R, Busemann H (2012) Noble gas chronology of EH5 chondrite St. Mark’s: an in-vacuo etch experiment. Meteor Planet Sci Suppl 47:5335
Craig JR, Barton PB Jr (1973) Thermochemical approximations for sulfosalts. Econ Geol 68:493–506
Czamanske GK, Erd RC, Sokolova NM, Dobrovol’skaya MG, Dmitrievea MT (1979) New data on rasvumite and djerfisherite. Am Mineral 64:776–778
Desch SJ, Connolly HC Jr (2002) A model of the thermal processing of particles in solar nebula shocks: application to the cooling rates of chondrules. Meteor Planet Sci 37:183–207
Dmitrievea MT, Hyukhin VV (1975) Crystal structure of djerfisherite. Doklady Akademiia Nauk USSR 223: 343–346 (transl. Soviet Physics Doklady 20:469–470, 1976)
Ebel DS (2006) Condensation of rocky material in astrophysical environments. In: Lauretta DS, McSween H (eds) Meteorites and the early solar system II. University of Arizona, Tucson, pp 253–277
Ebel DS, Alexander CMO’D (2011) Equilibrium condensation from chondritic porous IDP enriched vapor: implications for Mercury and enstatite chondrite origins. Planet Space Sci 59:1888–1894
Ebel DS, Grossman L (2000) Condensation in dust-enriched systems. Geochim Cosmochim Acta 64:339–366
Ebel DS, Sack RO (1994) Experimental determination of the free energy of formation of freibergite fahlore. Geochim Cosmochim Acta 58:1237–1242
El Goresy A, Grögler N, Ottemann J (1971) Djerfisherite composition in Bishopville, Peña Blanca Springs, St. Marks and Toluca Meteorites. Chem Erde 30:77–82
El Goresy A, Hideo Y, Ehlers K, Woolum D, Pernicka E (1988) Qingzhen and Yamato-691: a tentative alphabet for the EH Chondrites. Proc NIPR Symp Antarct Meteorites 1:65–101
Evans HT Jr, Clark JR (1981) The crystal structure of bartonite, a potassium iron sulfide, and its relationship to pentlandite and djerfisherite. Am Mineral 66:376–384
Fegley B Jr, Cameron AGW (1987) A vaporization model for iron/silicate fractionation in the Mercury protoplanet. Earth Planet Sci Lett 82:207–222
Fuchs LH (1966) Djerfisherite, alkali-copper sulfide: a new mineral from enstatite chondrites. Science 153:166–167
Goettel KA (1976) Models for the origin and composition of the earth, and the hypothesis of potassium in the earth’s core. Surv Geophys 2:369–397
Goettel KA (1988) Present bounds on the bulk composition of mercury: implications for planetary formation processes. In: Vilas F, Chapman CR, Matthews MS (eds) Mercury. University of Arizona, Tucson, pp 613–621
Henderson CMB, Kogarko LN, Plant DA (1999) Extreme closed system fractionation of volatile-rich, ultrabasic peralkaline melt inclusions and the occurrence of djerfisherite in the Kugda alkaline complex, Siberia. Mineral Mag 63:433–438
Javoy M (1995) The integral enstatite chondrite model of the Earth. Geophys Res Lett 22:2219–2222
Keil K (1968) Mineralogical and chemical relationships among enstatite chondrites. J Geophys Res 73:6945–6976
Keil K (1989) Enstatite meteorites and their parent bodies. Meteoritics 24:195–208
Keil K (2010) Enstatite achondrite meteorites (aubrites) and the histories of their asteroidal parent bodies. Chem Erde 70:395–417
Kimura M (1988) Origin of opaque minerals in an unequilibrated enstatite chondrite, Yamato-691. Proc NIPR Symp Antarctic Meteorites 1:51–64
Kracher A, Kurat G, Buchwald VF (1977) Cape York; The extraordinary mineralogy of an ordinary iron meteorite and its implication for the genesis of IIIAB irons. Geochem J 11:207–217
Lehner SW, Buseck PR, McDonough WF (2010) Origin of kamacite, schreibersite, and perryite in metal-sulfide nodules of the enstatite chondrite Sahara 97072 (EH3). Meteor Planet Sci 45:289–303
Lewis JS (1971) Consequences of the presence of sulfur in the core of the Earth. Earth Planet Sci Lett 11:130–134
Lin Y, El Goresy A (2002) A comparative study of opaque phases in Qingzhen (EH3) and MacAlpine Hills 88136: representatives of EH and EL parent bodies. Meteor Planet Sci 37:577–599
Lodders K (1995) Alkali elements in the earth’s core: evidence from enstatite meteorites. Meteor Planet Sci 30:93–101
Lodders K (2003) Solar system abundances and condensation temperatures of the elements. Astrophys J 591:1220–1247
Lodders K, Fegley B Jr (1998) The planetary scientist’s companion. Oxford University Press, New York
McCoy TJ, Dickinson TL, Lofgren GE (1999) Partial melting of the Indarch (EH4) meteorite: a textural, chemical and phase relations view of melting and melt migration. Meteor Planet Sci 34:735–746
McDonough WF (2003) Compositional model for the Earth’s core. In: Carlson RW (ed) The mantle and core, pp 547–568, In: Holland HD, Turekian KK (eds) Treatise on geochemistry. Elsevier, vol 2
McDonough WF, Sun S–S (1995) The composition of the Earth. Chem Geol 120:223–253
McNally C, Hubbard A, Mac Low M-M, Ebel DS, D’Alessio P (2013) Mineral processing by short-circuits in protoplanetary disks. Astrophys J (in press; http://arxiv.org/pdf/1301.1698.pdf)
Nittler LR, Starr RD, Weider SZ, McCoy TJ, Boynton WV, Ebel DS, Ernst CM, Evans LG, Goldsten JO, Hamara DK, Lawrence DJ, McNutt RL Jr, Schlemm CE II, Solomon SC, Sprague AL (2011) The major-element composition of Mercury’s surface from MESSENGER x-ray spectrometry. Science 333:1847–1850
Peplowski PN, Evans LG, Hauck SA II, McCoy TJ, Boynton WV, Gillis-Davis J, Ebel DS, Goldsten JO, Hamara DK, Lawrence DJ, McNutt RL Jr, Nittler LR, Solomon SC, Rhodes EA, Sprague AL, Starr RD, Stockstill-Cahill KR (2011) Radioactive elements on Mercury’s surface from MESSENGER: implications for the planet’s formation and evolution. Science 333:1850–1852
Rajamani V, Prewitt CT (1973) Crystal chemistry of natural pentlandites. Can Mineral 12:178–187
Rama Murthy V, van Westrenen W, Fei Y (2003) Experimental evidence that potassium is a substantial radioactive heat source in planetary cores. Nature 423:163–165
Rietmeijer FJM (2002) The earliest chemical dust evolution in the solar nebula. Chem Erde 62:1–45
Robinson MS, Taylor GJ (2001) Ferrous oxide in Mercury’s crust and mantle. Meteor Planet Sci 36:841–847
Sack RO, Ebel DS (2006) Thermochemistry of sulfide mineral solutions. Rev Mineral Geochem 61:265–364
Sack RO, Lichtner PC (2009) Constraining compositions of hydrothermal fluids in equilibrium with polymetallic ore forming sulfide assemblages. Econ Geol 104:1249–1264
Sack RO, Ebel DS, O’Leary MJ (1987) Tennahedrite thermochemistry and metal zoning. In Helgeson HC (ed) Chemical transport in metasomatic processes. D. Reidel, Dordrecht, Holland, pp 701–731
Sharygin VV, Golovin AV, Pokhilenko NP, Kamenetsky WS (2007) Djerfisherite Udachnaya-East pipe kimberlites (Sakha-Yakutia, Russia): paragenesis, composition and origin. Eur J Mineral 19:51–63
Sommerville M, Ahrens TJ (1980) Shock compression of KFeS2 and the question of potassium in the core. J Geophys Res 85:7016–7024
Tani BS (1977) X-ray study of K6LiFe24S26Cl, a djerfisherite-like compound. Am Mineral 62:819–923
Thompson JB Jr (1969) Chemical reactions in crystals. Am Mineral 54:341–375
Urey HC (1955) The cosmic abundances of potassium, uranium, and the heat balance of the earth, the moon, and Mars. Proc Nat Acad Sci 41:127–144
van Acken D, Humayun M, Brandon AD, Peslier AH (2012) Siderophile trace elements in metals and sulfides in enstatite achondrites record planetary differentiation in an enstatite chondritic parent body. Geochim Cosmochim Acta 83:272–291
van Westrenen W, Rama Murthy V (2006) Bulk Earth compositional models are consistent with the presence of potassium in Earth’s core. Workshop early planet differentiation, LPI. pp 113–114. http://www.lpi.usra.edu/lpi/contribution_docs/LPI-001335.pdf
Wasserburg GJ, MacDonald GJF, Hoyle F, Fowler WA (1964) Relative contributions of Uranium, Thorium, and Potassium to heat production in the Earth. Science 143:465–467
Wasson JT, Kallemeyn GW (1988) Composition of chondrites. Phil Trans R Soc London A 325:535–544
Watters TR, Prinz M (1979) Aubrites: their origin and relationship to enstatite chondrites. Proc Lunar Planet Sci Conf 10:1073–1093
Weidenschilling SJ (1978) Iron/silicate fractionation and the origin of Mercury. Icarus 35:99–111
Weider SZ, Nittler LR, Starr RD, McCoy TJ, Stockstill-Cahill KR, Byrne PK, Denevi BW, Head JW, Solomon SC (2012) Chemical heterogeneity on Mercury’s surface revealed by the MESSENGER x-ray spectrometer. J Geophys Res 117:E00L05. doi:10.1029/2012JE004153
Weisberg MK, Kimura M (2012) The unequilibrated enstatite chondrites. Chem Erde 72:101–115
Zaccarini F, Thalhammer OAR, Princivalle F, Lenaz D, Stanley CJ, Garuti G (2007) Djerfisherite in the Guli dunite complex, polar Siberia: a primary or metasomatic phase? Can Mineral 45:1201–1221
Acknowledgments
This manuscript was greatly improved by reviews from Dr. A. El Goresy and an anonymous reviewer. This research has made use of the National Aeronautics and Space Administration’s Astrophysics Data System Bibliographic Services. Research was supported by the American Museum of Natural History, National Aeronautics and Space Administration grant NNX10AI42G (DSE), and the friendly staff of OFM Research (ROS). Dr. Y. Lin kindly shared complete EPMA analyses for Qingzhen djerfisherites. We wish to dedicate this paper to Dr. Ahmed El Goresy upon his acceptance of the Leonard Medal of the Meteoritical Society in 2013, and to Dr. Ian Carmichael, whose interest in potassic igneous rocks was certainly not earthbound.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. L. Grove.
Appendices
Appendix 1: ordering calculation
defining \( {\text{K}}_{ 1} \equiv { \exp }\left( {\frac{25}{48}\Updelta \bar{G}_{Ni - Fe}^{{{\text{o - }}ORD}} /RT} \right) \), and \( {\text{D}} \equiv {\text{X}}_{ 2} -\left( {{\text{X}}_{ 2} } \right)^{ 2} -{\text{X}}_{ 2} {\text{X}}_{ 3} + \frac{1}{25}{\text{X}}_{ 2} {\text{s}}_{ 2} \) and \( {\text{E}} \equiv \frac{24}{25}( 1-{\text{X}}_{ 3} + \frac{1}{25} {\text{s}}_{ 2} )-\frac{23}{25} {\text{X}}_{ 2} , \) and \( {\text{F}} \equiv \frac{24}{625} \), and \( {\text{X}} \equiv {\text{X}}_{ 2} -\left( {{\text{X}}_{ 2} } \right)^{ 2} -{\text{X}}_{ 2} {\text{X}}_{ 3} -\frac{24}{25}{\text{X}}_{ 2}{\text{s}}_{ 2} , \) and \( {\text{Y}} \equiv \frac{1}{25}(- 1- 2 3 {\text{ X}}_{ 2} + {\text{X}}_{ 3} + \frac{24}{25} {\text{s}}_{ 2} ), \) and \( {\text{Z}} \equiv \frac{24}{625} \) we have \( 0 = \left( {{\text{K}}_{ 1} {\text{D}} - {\text{X}}} \right) + \left( {{\text{K}}_{ 1} {\text{E}} - {\text{Y}}} \right){\text{s}}_{ 1} + \left( {{\text{K}}_{ 1} {\text{F}} - {\text{Z}}} \right)\left( {{\text{s}}_{ 1} } \right)^{ 2} \).
Substituting X2 for X3, and X3 for X2, and s1 for s2, and s2 for s1, and \( \Updelta \bar{G}_{Cu - Fe}^{{{\text{o - }}ORD}} \) for \( \Updelta \bar{G}_{Ni - Fe}^{{{\text{o - }}ORD}} \) in the first expression above, leads to an analogous quadratic equation in s2 in similarly substituted variables.
Appendix 2: EPMA method
Electron probe microanalysis was performed with one micron spot size, at 15 keV accelerating voltage and 20 nA beam current. Natural and synthetic standards were used to calibrate on the Kα lines for 16 elements using wavelength dispersive spectrometers (WDS) and one element using energy dispersive analysis (EDS). Unknowns were analyzed in five passes as listed in Table 4, and an EDS spectrum was collected on each spot with a 240 s dwell time.
Rights and permissions
About this article
Cite this article
Ebel, D.S., Sack, R.O. Djerfisherite: nebular source of refractory potassium. Contrib Mineral Petrol 166, 923–934 (2013). https://doi.org/10.1007/s00410-013-0898-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00410-013-0898-x