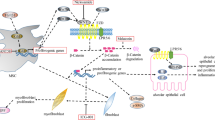
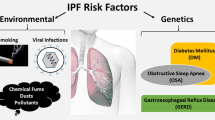
Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive and irreversible pulmonary interstitial disease that seriously affects the patient’s quality of life and lifespan. The pathogenesis of IPF has not been clarified, and its treatment is limited to pirfenidone and nintedanib, which only delays the decline of lung function. Alveolar epithelial type 2 (AT2) cells are indispensable in the regeneration and lung surfactant secretion of alveolar epithelial cells. Studies have shown that AT2 cell dysfunction initiates the occurrence and progression of IPF. This review expounds on the AT2 cell dysfunction in IPF, involving senescence, apoptosis, endoplasmic reticulum stress, mitochondrial damage, metabolic reprogramming, and the transitional state of AT2 cells. This article also briefly summarizes potential treatments targeting AT2 cell dysfunction.


Similar content being viewed by others
References
Lederer DJ, Martinez FJ (2018) Idiopathic pulmonary fibrosis. N Engl J Med 379(8):797–798. https://doi.org/10.1056/NEJMc1807508
Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J et al (2015) An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med 192(2):3–19
Olson AL, Gifford AH, Inase N, Fernandez PER, Suda T (2018) The epidemiology of idiopathic pulmonary fibrosis and interstitial lung diseases at risk of a progressive-fibrosing phenotype. Eur Respir Rev 27(150):180077
Parimon T, Yao C, Stripp BR, Noble PW, Chen P (2020) Alveolar epithelial type II cells as drivers of lung fibrosis in idiopathic pulmonary fibrosis. Int J Mol Sci 21(7):2269
Alysandratos K-D, Russo SJ, Petcherski A, Taddeo EP, Acin-Perez R, Villacorta-Martin C et al (2021) Patient-specific iPSCs carrying an SFTPC mutation reveal the intrinsic alveolar epithelial dysfunction at the inception of interstitial lung disease. Cell Rep. https://doi.org/10.1016/j.celrep.2021.109636
Tan W, Wang Y, Chen Y, Chen C (2021) Cell tracing reveals the transdifferentiation fate of mouse lung epithelial cells during pulmonary fibrosis in vivo. Exp Ther Med. https://doi.org/10.3892/etm.2021.10622
Alder JK, Barkauskas CE, Limjunyawong N, Stanley SE, Kembou F, Tuder RM et al (2015) Telomere dysfunction causes alveolar stem cell failure. Proc Natl Acad Sci USA 112(16):5099–5104. https://doi.org/10.1073/pnas.1504780112
Chen H, Chen H, Liang J, Gu X, Zhou J, Xie C et al (2020) TGF-beta1/IL-11/MEK/ERK signaling mediates senescence-associated pulmonary fibrosis in a stress-induced premature senescence model of Bmi-1 deficiency. Exp Mol Med 52(1):130–151
Yao C, Guan X, Carraro G, Parimon T, Liu X, Huang G et al (2021) Senescence of alveolar type 2 cells drives progressive pulmonary fibrosis. Am J Respir Crit Care Med 203(6):707–717. https://doi.org/10.1164/rccm.202004-1274OC
Yang L, Wang Y, Pan Z, Gao S, Zou B, Lin Z et al (2018) Tetraspanin 1 inhibits TNFα-induced apoptosis via NF-κB signaling pathway in alveolar epithelial cells. Inflamm Res 67(11–12):951–964. https://doi.org/10.1007/s00011-018-1189-9
Jiang C, Liu G, Cai L, Deshane J, Antony V, Thannickal VJ et al (2021) Divergent regulation of alveolar type 2 cell and fibroblast apoptosis by plasminogen activator inhibitor 1 in lung fibrosis. Am J Pathol 191(7):1227–1239. https://doi.org/10.1016/j.ajpath.2021.04.003
Borok Z, Horie M, Flodby P, Wang H, Liu Y, Ganesh S et al (2020) Grp78 loss in epithelial progenitors reveals an age-linked role for endoplasmic reticulum stress in pulmonary fibrosis. Am J Respir Crit Care Med 201(2):198–211
Tat V, Ayaub EA, Ayoub A, Vierhout M, Naiel S, Padwal MK et al (2021) FK506-binding protein 13 expression is upregulated in interstitial lung disease and correlated with clinical severity. A potentially protective role. Am J Respir Cell Mol Biol 64(2):235–246. https://doi.org/10.1165/rcmb.2020-0121OC
Bueno M, Brands J, Voltz L, Fiedler K, Mays B et al (2018) ATF3 represses PINK1 gene transcription in lung epithelial cells to control mitochondrial homeostasis. Aging Cell 17(2):e12720
Knoell J, Chillappagari S, Knudsen L, Korfei M, Dartsch R, Jonigk D et al (2022) PACS2-TRPV1 axis is required for ER-mitochondrial tethering during ER stress and lung fibrosis. Cell Mol Life Sci 79(3):151. https://doi.org/10.1007/s00018-022-04189-2
Zhang Y, Huang W, Zheng Z, Wang W, Yuan Y, Hong Q et al (2021) Cigarette smoke-inactivated SIRT1 promotes autophagy-dependent senescence of alveolar epithelial type 2 cells to induce pulmonary fibrosis. Free Radic Biol Med 166:116–127
Bindu S, Pillai VB, Kanwal A, Samant S, Mutlu GM, Verdin E et al (2017) SIRT3 blocks myofibroblast differentiation and pulmonary fibrosis by preventing mitochondrial DNA damage. Am J Physiol Lung Cell Mol Physiol 312(1):L68–L78. https://doi.org/10.1152/ajplung.00188.2016
Kim SJ, Cheresh P, Eren M, Jablonski RP, Yeldandi A, Ridge KM et al (2017) Klotho, an antiaging molecule, attenuates oxidant-induced alveolar epithelial cell mtDNA damage and apoptosis. Am J Physiol Lung Cell Mol Physiol 313(1):L16–L26
Wu H, Yu Y, Huang H, Hu Y, Fu S, Wang Z et al (2020) Progressive pulmonary fibrosis is caused by elevated mechanical tension on alveolar stem cells. Cell 180(1):107. https://doi.org/10.1016/j.cell.2019.11.027
Kobayashi Y, Tata A, Konkimalla A, Katsura H, Lee RF, Ou J et al (2020) Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nat Cell Biol 22(8):934–946. https://doi.org/10.1038/s41556-020-0542-8
Duerr J, Leitz DHW, Szczygiel M, Dvornikov D, Fraumann SG, Kreutz C et al (2020) Conditional deletion of Nedd4-2 in lung epithelial cells causes progressive pulmonary fibrosis in adult mice. Nat Commun 11(1):2012. https://doi.org/10.1038/s41467-020-15743-6
Guan S, Liu H, Zhou J, Zhang Q, Bi H (2022) The MIR100HG/miR-29a-3p/Tab1 axis modulates TGF-beta1-induced fibrotic changes in type II alveolar epithelial cells BLM-caused lung fibrogenesis in mice. Toxicol Lett. https://doi.org/10.1016/j.toxlet.2022.04.003
Ciechomska M, O’Reilly S, Suwara M, Bogunia-Kubik K, van Laar JM (2014) MiR-29a reduces TIMP-1 production by dermal fibroblasts via targeting TGF-beta activated kinase 1 binding protein 1, implications for systemic sclerosis. PLoS ONE 9(12):e115596. https://doi.org/10.1371/journal.pone.0115596
Yao L, Conforti F, Hill C, Bell J, Drawater L, Li J et al (2019) Paracrine signalling during ZEB1-mediated epithelial-mesenchymal transition augments local myofibroblast differentiation in lung fibrosis. Cell Death Differ 26(5):943–957. https://doi.org/10.1038/s41418-018-0175-7
Gokey JJ, Snowball J, Green J, Waltamath M, Spinney JJ, Black KE et al (2021) Pretreatment of aged mice with retinoic acid supports alveolar regeneration via upregulation of reciprocal PDGFA signalling. Thorax 76(5):456–467
Wilson C, Mertens TC, Shivshankar P, Bi W, Collum SD, Wareing N et al (2022) Sine oculis homeobox homolog 1 plays a critical role in pulmonary fibrosis. JCI Insight. https://doi.org/10.1172/jci.insight.142984
Tian Y, Lv J, Su Z, Wu T, Li X, Hu X et al (2021) LRRK2 plays essential roles in maintaining lung homeostasis and preventing the development of pulmonary fibrosis. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.2106685118
Young LR, Gulleman PM, Short CW, Tanjore H, Sherrill T, Qi A et al (2016) Epithelial–macrophage interactions determine pulmonary fibrosis susceptibility in Hermansky–Pudlak syndrome. JCI Insight 1(17):e88947. https://doi.org/10.1172/jci.insight.88947
Hou J, Ji J, Chen X, Cao H, Tan Y, Cui Y et al (2021) Alveolar epithelial cell-derived Sonic hedgehog promotes pulmonary fibrosis through OPN-dependent alternative macrophage activation. FEBS J 288(11):3530–3546. https://doi.org/10.1111/febs.15669
Gu L, Surolia R, Larson-Casey JL, He C, Davis D, Kang J et al (2022) Targeting Cpt1a-Bcl-2 interaction modulates apoptosis resistance and fibrotic remodeling. Cell Death Differ 29(1):118–132. https://doi.org/10.1038/s41418-021-00840-w
Armanios M (2013) Telomeres and age-related disease: how telomere biology informs clinical paradigms. J Clin Invest 123(3):996–1002. https://doi.org/10.1172/JCI66370
van Batenburg AA, Kazemier KM, van Oosterhout MFM, van der Vis JJ, Grutters JC, Goldschmeding R et al (2021) Telomere shortening and DNA damage in culprit cells of different types of progressive fibrosing interstitial lung disease. ERJ Open Res. https://doi.org/10.1183/23120541.00691-2020
Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR et al (2013) Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 123(7):3025–3036. https://doi.org/10.1172/JCI68782
Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A et al (2010) Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med 181(3):254–263. https://doi.org/10.1164/rccm.200810-1615OC
Garcia O, Hiatt MJ, Lundin A, Lee J, Reddy R, Navarro S et al (2016) Targeted type 2 alveolar cell depletion. A dynamic functional model for lung injury repair. Am J Respir Cell Mol Biol 54(3):319–330. https://doi.org/10.1165/rcmb.2014-0246OC
Pfaffenbach KT, Lee AS (2011) The critical role of GRP78 in physiologic and pathologic stress. Curr Opin Cell Biol 23(2):150–156
Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XC et al (2011) Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci USA 108(26):10562–10567. https://doi.org/10.1073/pnas.1107559108
Katzen J, Wagner BD, Venosa A, Kopp M, Tomer Y, Russo SJ et al (2019) An SFTPC BRICHOS mutant links epithelial ER stress and spontaneous lung fibrosis. JCI Insight. https://doi.org/10.1172/jci.insight.126125
Kim SJ, Cheresh P, Jablonski RP, Rachek L, Yeldandi A, Piseaux-Aillon R et al (2020) Mitochondrial 8-oxoguanine DNA glycosylase mitigates alveolar epithelial cell PINK1 deficiency, mitochondrial DNA damage, apoptosis, and lung fibrosis. Am J Physiol Lung Cell Mol Physiol 318(5):L1084–L1096
Simmen T, Aslan JE, Blagoveshchenskaya AD, Thomas L, Wan L, Xiang Y et al (2005) PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J 24(4):717–729. https://doi.org/10.1038/sj.emboj.7600559
Weng H, Ma Y, Chen L, Cai G, Chen Z, Zhang S et al (2020) A new vision of mitochondrial unfolded protein response to the sirtuin family. Curr Neuropharmacol 18(7):613–623. https://doi.org/10.2174/1570159X18666200123165002
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T et al (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390(6655):45–51
Mencke R, Hillebrands JL (2017) The role of the anti-ageing protein Klotho in vascular physiology and pathophysiology. Ageing Res Rev 35:124–146. https://doi.org/10.1016/j.arr.2016.09.001
Chen G, Liu Y, Goetz R, Fu L, Jayaraman S, Hu MC et al (2018) alpha-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature 553(7689):461–466
Erben RG (2018) alpha-Klotho’s effects on mineral homeostasis are fibroblast growth factor-23 dependent. Curr Opin Nephrol Hypertens 27(4):229–235
Shaghaghi H, Para R, Tran C, Roman J, Ojeda-Lassalle Y, Sun J et al (2021) Glutamine restores mitochondrial respiration in bleomycin-injured epithelial cells. Free Radic Biol Med 176:335–344. https://doi.org/10.1016/j.freeradbiomed.2021.10.006
Wang S, Li X, Ma Q, Wang Q, Wu J, Yu H et al (2022) Glutamine metabolism is required for alveolar regeneration during lung injury. Biomolecules. https://doi.org/10.3390/biom12050728
Yoo HC, Yu YC, Sung Y, Han JM (2020) Glutamine reliance in cell metabolism. Exp Mol Med 52(9):1496–1516. https://doi.org/10.1038/s12276-020-00504-8
Tran TQ, Lowman XH, Kong M (2017) Molecular pathways: metabolic control of histone methylation and gene expression in cancer. Clin Cancer Res 23(15):4004–4009. https://doi.org/10.1158/1078-0432.CCR-16-2506
Adams TS, Schupp JC, Poli S, Ayaub EA, Neumark N, Ahangari F et al (2020) Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv 6(28):eaba1983. https://doi.org/10.1126/sciadv.aba1983
Kathiriya JJ, Wang C, Zhou M, Brumwell A, Cassandras M, Le Saux CJ et al (2022) Human alveolar type 2 epithelium transdifferentiates into metaplastic KRT5(+) basal cells. Nat Cell Biol 24(1):10–23. https://doi.org/10.1038/s41556-021-00809-4
Strunz M, Simon LM, Ansari M, Kathiriya JJ, Angelidis I, Mayr CH et al (2020) Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat Commun 11(1):3559. https://doi.org/10.1038/s41467-020-17358-3
Wang Y, Liu J, Chen J, Feng T, Guo Q (2015) MiR-29 mediates TGFbeta 1-induced extracellular matrix synthesis through activation of Wnt/beta-catenin pathway in human pulmonary fibroblasts. Technol Health Care 23(Suppl 1):S119–S125. https://doi.org/10.3233/thc-150943
Yao L, Zhou Y, Li J, Wickens L, Conforti F, Rattu A et al (2021) Bidirectional epithelial–mesenchymal crosstalk provides self-sustaining profibrotic signals in pulmonary fibrosis. J Biol Chem 297(3):101096. https://doi.org/10.1016/j.jbc.2021.101096
El-Hashash AH, Al Alam D, Turcatel G, Rogers O, Li X, Bellusci S et al (2011) Six1 transcription factor is critical for coordination of epithelial, mesenchymal and vascular morphogenesis in the mammalian lung. Dev Biol 353(2):242–258. https://doi.org/10.1016/j.ydbio.2011.02.031
Naikawadi RP, Disayabutr S, Mallavia B, Donne ML, Green G, La JL et al (2016) Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight 1(14):e86704. https://doi.org/10.1172/jci.insight.86704
Gunther S, Fagone P, Jalce G, Atanasov AG, Guignabert C, Nicoletti F (2019) Role of MIF and D-DT in immune-inflammatory, autoimmune, and chronic respiratory diseases: from pathogenic factors to therapeutic targets. Drug Discov Today 24(2):428–439. https://doi.org/10.1016/j.drudis.2018.11.003
Gunther S, Bordenave J, Hua-Huy T, Nicco C, Cumont A, Thuillet R et al (2018) Macrophage migration inhibitory factor (MIF) inhibition in a murine model of bleomycin-induced pulmonary fibrosis. Int J Mol Sci. https://doi.org/10.3390/ijms19124105
Aumiller V, Balsara N, Wilhelm J, Günther A, Königshoff M (2013) WNT/β-catenin signaling induces IL-1β expression by alveolar epithelial cells in pulmonary fibrosis. Am J Respir Cell Mol Biol 49(1):96–104. https://doi.org/10.1165/rcmb.2012-0524OC
Baran CP, Opalek JM, McMaken S, Newland CA, O’Brien JM Jr, Hunter MG et al (2007) Important roles for macrophage colony-stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 176(1):78–89. https://doi.org/10.1164/rccm.200609-1279OC
Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK et al (2019) Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 40:554–563. https://doi.org/10.1016/j.ebiom.2018.12.052
Dahl TB, Holm S, Aukrust P, Halvorsen B (2012) Visfatin/NAMPT: a multifaceted molecule with diverse roles in physiology and pathophysiology. Annu Rev Nutr 32:229–243. https://doi.org/10.1146/annurev-nutr-071811-150746
Lai X, Huang S, Lin S, Pu L, Wang Y, Lin Y et al (2022) Mesenchymal stromal cells attenuate alveolar type 2 cells senescence through regulating NAMPT-mediated NAD metabolism. Stem Cell Res Ther 13(1):12. https://doi.org/10.1186/s13287-021-02688-w
Averyanov A, Koroleva I, Konoplyannikov M, Revkova V, Lesnyak V, Kalsin V et al (2020) First-in-human high-cumulative-dose stem cell therapy in idiopathic pulmonary fibrosis with rapid lung function decline. Stem Cells Transl Med 9(1):6–16. https://doi.org/10.1002/sctm.19-0037
Takao S, Nakashima T, Masuda T, Namba M, Sakamoto S, Yamaguchi K et al (2021) Human bone marrow-derived mesenchymal stromal cells cultured in serum-free media demonstrate enhanced antifibrotic abilities via prolonged survival and robust regulatory T cell induction in murine bleomycin-induced pulmonary fibrosis. Stem Cell Res Ther 12(1):506. https://doi.org/10.1186/s13287-021-02574-5
Xie T, Kulur V, Liu N, Deng N, Wang Y, Rowan SC et al (2021) Mesenchymal growth hormone receptor deficiency leads to failure of alveolar progenitor cell function and severe pulmonary fibrosis. Sci Adv. https://doi.org/10.1126/sciadv.abg6005
Tanaka Y, Ishitsuka Y, Hayasaka M, Yamada Y, Miyata K, Endo M et al (2015) The exacerbating roles of CCAAT/enhancer-binding protein homologous protein (CHOP) in the development of bleomycin-induced pulmonary fibrosis and the preventive effects of tauroursodeoxycholic acid (TUDCA) against pulmonary fibrosis in mice. Pharmacol Res 99:52–62. https://doi.org/10.1016/j.phrs.2015.05.004
Winters CJ, Koval O, Murthy S, Allamargot C, Sebag SC, Paschke JD et al (2016) CaMKII inhibition in type II pneumocytes protects from bleomycin-induced pulmonary fibrosis by preventing Ca2+-dependent apoptosis. Am J Physiol Lung Cell Mol Physiol 310(1):L86-94. https://doi.org/10.1152/ajplung.00132.2015
Yu G, Tzouvelekis A, Wang R, Herazo-Maya JD, Ibarra GH, Srivastava A et al (2018) Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med 24(1):39. https://doi.org/10.1038/nm.4447
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82000065), Beijing Key Clinical Specialty Construction Project (2020–2022).
Author information
Authors and Affiliations
Contributions
WZ conceived, wrote, and edited the manuscript. CT and JZ conceived and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, W., Tan, C. & Zhang, J. Alveolar Epithelial Type 2 Cell Dysfunction in Idiopathic Pulmonary Fibrosis. Lung 200, 539–547 (2022). https://doi.org/10.1007/s00408-022-00571-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-022-00571-w