Abstract
Introduction
The use of molecular biomarkers to guide lung cancer management has led to increasing frequency and amounts of tissue required for repeat lung biopsies. While patient safety and reporting of adverse events has been increasingly emphasized in recent decades, the safety of lung biopsies in patients with lung cancer has only been studied in small cohorts. We therefore analyzed adverse events in patients with lung cancer undergoing lung biopsies in the National Hospital Discharge Survey (NHDS) database.
Methods
Data were abstracted using ICD-9 lung cancer diagnosis (162.X) and lung biopsy procedure codes (33.20, 33.24, 33.25, 33.26, 33.27, 33.28) from 2001 to 2010. Agency for Healthcare Research and Quality (AHRQ) Patient-Safety Indicators (PSI) were used to identify hospital-acquired adverse events. Weighted analyses were performed using SAS version 9.4.
Results
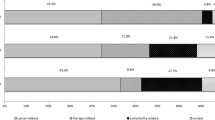
A total of 540,747 patients were included for analysis. The number of biopsies increased over time, from 51,221 in 2001, to 63,239 in 2010 (P < 0.001). Overall, 159,683 (30%) patients suffered ≥ 1-PSI event during their hospitalization. Incidence of PSI varied by biopsy type: bronchoscopic (26%), percutaneous (34%), surgical (39%). The proportion of patients with ≥ 1 PSI event increased from 24% in 2001 to 38% in 2010 (P < 0.001). Patients with ≥ 1 PSI had longer length of stay (mean, 11.6 vs 8.1 days; P < 0.001) and higher in-hospital mortality (adjusted odds ratio, 5.9, 95% CI 3.9–8.9; P < 0.001).
Conclusions
The frequency of lung biopsies performed and rate of documented adverse events in hospitalized lung cancer patients have increased. These findings have policy, funding, research, and practice implications.
Similar content being viewed by others
References
Piotrowska Z, Isozaki H, Lennerz JK, Gainor JF, Lennes IT, Zhu VW et al (2018) Landscape of acquired resistance to osimertinib in EGFR-mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU-667 for acquired RET fusion. Cancer Discov 8(12):1529–1539
Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P et al (2011) Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 3(75):75ra26.
Gainor JF, Dardaei L, Yoda S, Friboulet L, Leshchiner I, Katayama R et al (2016) Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 6(10):1118–1133
Rivard PE, Elwy AR, Loveland S, Zhao S, Tsilimingras D, Elixhauser A et al (2005) Applying patient safety indicators (PSIs) across health care systems: achieving data comparability. In: Henriksen K, Battles JB, Marks ES, Lewin DI (eds) Advances in patient safety: from research to implementation, vol. 2: concepts and methodology. Advances in Patient Safety, Rockville.
Chera BS, Mazur L, Buchanan I, Kim HJ, Rockwell J, Milowsky MI et al (2015) Improving patient safety in clinical oncology: applying lessons from normal accident theory. JAMA Oncol 1(7):958–964
Weaver SJ, Lubomksi LH, Wilson RF, Pfoh ER, Martinez KA, Dy SM (2013) Promoting a culture of safety as a patient safety strategy: a systematic review. Ann Intern Med 158(5 Pt 2):369–374
Parseghian CM, Tam AL, Yao J, Ensor J Jr, Ellis LM, Raghav K et al (2018) Assessment of reported trial characteristics, rate of publication, and inclusion of mandatory biopsies of research biopsies in clinical trials in oncology. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2018.4640
Heerink WJ, de Bock GH, de Jonge GJ, Groen HJ, Vliegenthart R, Oudkerk M (2017) Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 27(1):138–148
Wiener RS, Schwartz LM, Woloshin S, Welch HG (2011) Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med 155(3):137–144
Izbicki G, Shitrit D, Yarmolovsky A, Bendayan D, Miller G, Fink G et al (2006) Is routine chest radiography after transbronchial biopsy necessary? A prospective study of 350 cases. Chest 129(6):1561–1564
Asano F, Aoe M, Ohsaki Y, Okada Y, Sasada S, Sato S et al (2012) Deaths and complications associated with respiratory endoscopy: a survey by the Japan Society for Respiratory Endoscopy in 2010. Respirology 17(3):478–485
Tukey MH, Wiener RS (2012) Population-based estimates of transbronchial lung biopsy utilization and complications. Respir Med 106(11):1559–1565
Huo J, Xu Y, Sheu T, Volk RJ, Shih YT (2019) Complication rates and downstream medical costs associated with invasive diagnostic procedures for lung abnormalities in the community setting. JAMA Intern Med. https://doi.org/10.1001/jamainternmed.2018.6277
National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD et al (2011) Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 365(5), 395–409.
Liao BC, Bai YY, Lee JH, Lin CC, Lin SY, Lee YF et al (2018) Outcomes of research biopsies in clinical trials of EGFR mutation-positive non-small cell lung cancer patients pretreated with EGFR-tyrosine kinase inhibitors. J Formos Med Assoc 117(4):326–331
Yoon HJ, Lee HY, Lee KS, Choi YL, Ahn MJ, Park K et al (2012) Repeat biopsy for mutational analysis of non-small cell lung cancers resistant to previous chemotherapy: adequacy and complications. Radiology 265(3):939–948
Bach PB, Cramer LD, Schrag D, Downey RJ, Gelfand SE, Begg CB (2001) The influence of hospital volume on survival after resection for lung cancer. N Engl J Med 345(3):181–188
Onega T, Duell EJ, Shi X, Demidenko E, Goodman D (2009) Determinants of NCI Cancer Center attendance in Medicare patients with lung, breast, colorectal, or prostate cancer. J Gen Intern Med 24(2):205–210
Tsai J, Grosse SD, Grant AM, Reyes NL, Hooper WC, Atrash HK (2012) Correlates of in-hospital deaths among hospitalizations with pulmonary embolism: findings from the 2001–2008 National Hospital Discharge Survey. PLoS ONE 7(7):e34048
Wong CA, Jim MA, King J, Tom-Orme L, Henderson JA, Saraiya M et al (2011) Impact of hysterectomy and bilateral oophorectomy prevalence on rates of cervical, uterine, and ovarian cancer among American Indian and Alaska Native women, 1999–2004. Cancer Causes Control. 22(12):1681–1689
Memtsoudis SG, Besculides MC, Zellos L, Patil N, Rogers SO (2006) Trends in lung surgery: United States 1988 to 2002. Chest 130(5):1462–1470
Gupta A, Tariq R, Frank RD, Jean GW, Beg MS, Pardi DS et al (2017) Trends in the incidence and outcomes of hospitalized cancer patients with clostridium difficile infection: a nationwide analysis. J Natl Compr Canc Netw 15(4):466–472
Gupta A, Das A, Tariq R, Bhulani N, Premnath N, Solanky D et al (2018) Trends in outcomes of patients with metastatic cancer undergoing intubation and mechanical ventilation: results of the national hospital discharge survey. J Natl Compr Canc Netw 16(3):286–292
https://www.qualityindicators.ahrq.gov/Modules/PSI_TechSpec.aspx. Agency for healthcare research and quality: patient safety indicators technical specifications. Last Accessed 3 Feb 2019
Vincent C, Aylin P, Franklin BD, Holmes A, Iskander S, Jacklin A et al (2008) Is health care getting safer? BMJ 337:a2426
Downey JR, Hernandez-Boussard T, Banka G, Morton JM (2012) Is patient safety improving? National trends in patient safety indicators: 1998–2007. Health Serv Res 47(1 Pt 2):414–430
Levinson DR (2008) Adverse events in hospitals: an overview of key issues. DHHS Publication No. OEI-06-07-00470. Office of the Inspector General, Department of Health and Human Services, Washington, DC
Nguyen MC, Moffatt-Bruce SD, Van Buren A, Gonsenhauser I, Eiferman DS (2018) Daily review of AHRQ patient safety indicators has important impact on value-based purchasing, reimbursement, and performance scores. Surgery 163(3):542–546
Weissman JS, Lopez L, Schneider EC, Epstein AM, Lipsitz S, Weingart SN (2014) The association of hospital quality ratings with adverse events. Int J Qual Health Care 26(2):129–135
Sukumar S, Roghmann F, Trinh VQ, Sammon JD, Gervais MK, Tan HJ et al (2013) National trends in hospital-acquired preventable adverse events after major cancer surgery in the USA. BMJ Open 3(6):e002843
Narain W (2017) Assessing estimates of patient safety derived from coded data. J Healthc Qual. 39(4):230–242
U.S. Department of Health and Human Services CfDCaPaNCI (1999–2016) United States cancer statistics: data visualizations. changes over time: lung and bronchus U.S. Cancer Statistics Data Visualizations Tool, based on November 2018 submission data. www.cdc.gov/cancer/dataviz
Acknowledgements
The authors thank Helen Mayo, MLS from the UT Southwestern Medical Library for assistance with literature searches. The authors thank Ms. Dru Gray for assistance with manuscript preparation.
Funding
Funded in part by a National Cancer Institute Midcareer Investigator Award in Patient-Oriented Research (K24 CA201543-01; to DEG). This work is not under consideration for publication elsewhere. If accepted, this work will not be published elsewhere without written consent of the copyright-holder.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
von Itzstein, M.S., Gupta, A., Mara, K.C. et al. Increasing Numbers and Reported Adverse Events in Patients with Lung Cancer Undergoing Inpatient Lung Biopsies: A Population-Based Analysis. Lung 197, 593–599 (2019). https://doi.org/10.1007/s00408-019-00255-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-019-00255-y