Abstract
Background
Plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) levels are elevated in patients with secondary pulmonary hypertension and chronic lung disease with right ventricular overload. The aim of the present study was to investigate the use of plasma NT-proBNP levels as a prognostic marker of severe COPD with chronic respiratory failure and latent pulmonary hypertension.
Methods
Plasma NT-proBNP levels were measured in 61 patients with stable COPD. Plasma NT-proBNP levels, pulmonary function, PaO2, and PaCO2 levels and systolic pulmonary artery pressure were compared according to COPD severity. In addition, we examined correlations between plasma NT-proBNP levels and pulmonary function, PaO2, PaCO2, and systolic pulmonary artery pressure.
Results
The levels of plasma NT-proBNP significantly increased in patients with stage IV and stage III COPD compared to individuals with stage II COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification. The area under the receiver-operating characteristic curve of plasma NT-proBNP for severe to very severe COPD (FEV1 < 50%) was 0.707 (95% confidence interval [CI] 0.566–0.847, P = 0.008). Plasma NT-proBNP levels significantly correlated with %FEV1 (r = −0.557; P < 0.001), arterial blood gas parameters such as PaCO2 (r = 0.476; P < 0.001) and PaO2 (r = −0.347; P = 0.031), and systolic pulmonary artery pressure (r = 0.435; P = 0.001).
Conclusions
Plasma NT-proBNP levels increased significantly with disease severity, progression of chronic respiratory failure, and secondary pulmonary hypertension in patients with stable COPD. These results suggest that plasma NT-proBNP can be a useful prognostic marker to monitor COPD progression and identify cases of secondary pulmonary hypertension in patients with stable COPD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain natriuretic peptide (BNP) was first described in 1988 after being isolated from porcine brain [1]. However, this protein was soon found to originate mainly from the heart and identified as a cardiac hormone. BNP is synthesized as a prehormone (proBNP) composed of 108 amino acids. Upon release into circulation it is cleaved into equal amounts of biologically active 32-amino-acid BNP, which represents the C-terminal fragment, and the biologically inactive 76-amino-acid N-terminal fragment (NT-proBNP). The main stimulus responsible for increasing BNP and NT-proBNP synthesis and secretion is myocardial wall stress. Furthermore, factors such as myocardial ischemia and endocrine (paracrine) modulation by other neurohormones and cytokines are also involved. The half-life of BNP is 20 min, whereas NT-proBNP has a half-life of 120 min. This explains why NT-proBNP serum values are approximately six times higher than BNP values, even though both molecules are released in equimolar proportions.
Several large trials have found that the most frequent cause of death among patients with COPD is cardiac, rather than respiratory, complications [2, 3]. Secondary pulmonary hypertension and cor pulmonale are also important causes of death and poor prognosis in COPD patients [4, 5], so early detection of pulmonary hypertension or cor pulmonale could be beneficial in managing patients with COPD. BNP has been shown to be useful for the diagnosing patients suspected of having heart failure [6, 7]. It is also well known that plasma BNP levels are elevated in patients with secondary pulmonary hypertension and chronic lung disease with right ventricular overload [8–11]. However, few studies have assessed the diagnostic utility of NT-proBNP for determining COPD severity and identifying chronic respiratory failure with the possibility of progression to secondary pulmonary hypertension in patients with COPD. Therefore, the aim of the present study was to investigate the use of plasma NT-proBNP levels as a prognostic marker of severe COPD with chronic respiratory failure and latent pulmonary hypertension.
Materials and Methods
Subjects
Patients diagnosed with COPD according to the American Thoracic Society (ATS) guidelines [12] were recruited for this study from January to December 2010. The inclusion criteria were a history of smoking, a forced expiratory volume in 1 sec (FEV1)/forced vital capacity (FVC) ratio <70%, and an FEV1 <80% of the predicted values. Different conditions, including advanced age, gender, body mass index (BMI), and noncardiac diseases such as pulmonary and renal dysfunction and sepsis, might increase levels of plasma NT-proBNP [13–15] so we excluded patients with respiratory disorders other than COPD, pulmonary embolisms, infectious diseases, malignancy, left ventricular dysfunction (systolic and/or diastolic dysfunction), recent surgery, and severe endocrine, hepatic, or renal dysfunction. At recruitment, the patients’ height and weight were measured and body mass index was calculated (kg/m2). Disease severity was categorized according to GOLD guidelines and based on post-bronchodilator FEV1 (% predicted) values: stage I (mild), FEV1 ≥ 80% predicted; stage II (moderate), 50% ≤ FEV1 < 80% predicted; stage III (severe), 30% ≤ FEV1 < 50% predicted; and stage IV (very severe), FEV1 < 30% predicted or FEV1 < 50% predicted plus chronic respiratory failure. This study protocol was approved by the Chonnam National University Hospital Ethics Committee, and all subjects provided informed written consent prior to their participation.
Pulmonary Function Test
All pulmonary function tests were performed using a Sensormedics 2400 Unit (Sensormedics, San Diego, CA, USA) that met ATS standards [12]. Most pulmonary function tests were performed on the same day as the echocardiography and/or laboratory investigations. Three technically acceptable measurements were obtained for each patient, with the highest one included in the analyses. Acceptable test results were defined as those with a sharp peak in the flow curve and an expiratory duration greater than 6 s. FEV1, FVC, and lung volume were expressed as a percentage of the predicted values.
Echocardiography
Echocardiography was performed using a commercially available instrument (Siemens Medical Solution, Malvern, PA, USA) equipped with a 2.5-MHz probe. Tricuspid regurgitation flow was identified by color flow Doppler techniques, and the maximal jet velocity was recorded from the parasternal or apical window with a continuous wave Doppler echocardiographic probe. The trans-tricuspid pressure gradient (△P) could be calculated from the maximum velocity of the tricuspid regurgitant jets (V max) using the simplified form of the Bernoulli equation: △P (mmHg) = V max (m/s) × 4. An estimation of pulmonary artery pressure was obtained by adding an estimate of the right atrial pressure to △P [16]. The left ventricular ejection fraction (LVEF) was calculated using Simpson’s rule (disc summation method) [17]. Left ventricular systolic dysfunction was defined as LVEF < 50%. LV diastolic function was assessed by pulsed-wave Doppler mitral inflow and pulmonary venous inflow, and the E/A velocity ratio and ratio of systolic to diastolic forward flow (S/D ratio) were calculated. LV diastolic dysfunction was defined as impaired relaxation (grade I), pseudonormal filling (grade II), or restrictive filling (grade III) by a combination of transmitral and pulmonary flow patterns and left atrial volume indexes [18].
Laboratory Data
Blood samples for measuring plasma NT-proBNP levels were obtained by collecting peripheral blood in tubes containing ethylenediamine tetra-acetic acid (EDTA). Measurements were performed within 2 h of collection using a commercial kit (Roche Diagnostics Corp., Indianapolis, IN, USA) and an electrochemiluminescent method with an Elecsys 2010 Automated Analyzer (Roche Diagnostics). The results are presented as pg/ml. Serum creatinine (SCr) levels assays were all performed with the Jaffe method on a Hitachi Modular Pre-Analytics Plus system (Roche Diagnostics) and expressed as mg/dl. For each patient, renal function was evaluated by estimating glomerular filtration rate (GFR) with the MDRD Study equation [19]. Renal dysfunction was defined as GFR < 60 ml/min/1.73 m2.
Statistical Analysis
SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Data are expressed as the median (25th to 75th percentiles). To examine differences between the two groups, a Mann–Whitney test was used to compare continuous variables. To compare differences among the three groups, a Kruskal–Wallis test for continuous variables was performed. Correlation coefficients were calculated with the Spearman rank test. Receiver-operating characteristic (ROC) curve analysis was performed for plasma NT-proBNP levels, with the severity of COPD (GOLD stage II vs. GOLD stage III–IV) as the reference standard. A P value of <0.05 was considered to be statistically significant.
Results
The baseline characteristics of the study population are summarized in Table 1. Our study included 61 patients with stable COPD. Twenty-one patients were classified as having moderate COPD, 33 patients had severe COPD, and 7 patients were found to have very severe COPD when stratified by ATS guidelines. There were no significant differences in age, gender, smoking history (pack-years), BMI, LVEF, or GFR among subjects with different stages of COPD.
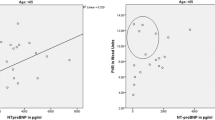
The levels of plasma NT-proBNP were significantly lower in patients with stage II COPD [112.0 (75.0–160.0) pg/ml] compared to individuals with stage III [151.0 (108.5–318.5) pg/ml, P = 0.018] and stage IV COPD [250.0 (201.0–371.0) pg/ml, P = 0.027] according to GOLD (Fig. 1a). However, no significant differences in plasma NT-proBNP levels were observed between patients with stage III and stage IV disease (P = 0.310) (Fig. 1a). If the patients were grouped as having moderate COPD (FEV1 ≥ 50%) and severe to very severe COPD (FEV1 < 50%), the levels of plasma NT-proBNP were significantly lower in patients with moderate COPD [112.0 (75.0–160.0) pg/ml] compared to severe to very severe COPD [166.0 (114.5–318.7) pg/ml, P = 0.008] (Fig. 1b). A receiver-operating characteristic curve for plasma NT-proBNP was performed for predicting severe to very severe COPD (FEV1 < 50%) in all patients (Fig. 1c). The area under the ROC curve of plasma NT-proBNP for severe to very severe COPD was 0.707 (95% confidence interval [CI] 0.566–0.847, P = 0.008). Plasma NT-proBNP levels significantly correlated with pulmonary function parameters such as %FEV1 (r = −0.557; P < 0.001) and arterial blood gas parameters such as PaCO2 (r = 0.476; P < 0.001) and PaO2 (r = –0.347; P = 0.031). There was also a significant positive correlation between plasma NT-proBNP concentrations and systolic pulmonary artery pressure (r = 0.435; P = 0.001) (Fig. 2).
a The levels of plasma NT-proBNP were significantly lower in patients with stage II COPD (moderate COPD) compared to individuals with stage III (severe) and stage IV (very severe) COPD according to GOLD classification. b If the patients are grouped as having moderate COPD (FEV1 ≥ 50%) and severe to very severe COPD (FEV1 < 50%), the levels of plasma NT-proBNP are significantly lower in patients with moderate COPD compared to severe to very severe COPD. Stage II, 50% ≤ FEV1 < 80% predicted; stage III, 30% ≤ FEV1 < 50% predicted; stage IV, FEV1 < 30% predicted or FEV1 < 50% predicted plus chronic respiratory failure. a, b Data are shown as box plots. Each box represents the 25th to 75th percentiles. Lines inside the boxes represent the median. Whiskers represent the 10th and 90th percentiles. c A receiver operator characteristic (ROC) curve of plasma NT-proBNP as a predictor of severe to very severe COPD (FEV1 < 50%) in overall COPD patients
Correlations between plasma NT-proBNP levels and % FEV1, systolic pulmonary artery pressure, arterial oxygen tension (PaO2), and arterial carbon dioxide tension (PaCO2) in patients with COPD. a Negative correlation between NT-proBNP levels and FEV1 % predicted (r = −0.557, p < 0.001). b Positive correlation between NT-proBNP levels and systolic pulmonary artery pressure (r = 0.435, P = 0.001). c Negative correlation between NT-proBNP levels and PaO2 (r = −0.347, P = 0.031). d Positive correlation between NT-proBNP levels and PaCO2 (r = 0.476, P < 0.001)
Discussion
In the present study, we specifically examined the potential of plasma NT-proBNP levels in patients with stable COPD to predict the severity of COPD, chronic respiratory failure, and latent secondary pulmonary hypertension. Here we report three major findings. First, we determined that plasma NT-proBNP levels in patients with stable COPD increased significantly with disease severity. The levels of plasma NT-proBNP were significantly higher in patients with stage IV and stage III COPD compared to individuals with stage II COPD. Figure 1a shows that the level of plasma NT-proBNP in patients with stage IV COPD is not significantly different from that of individuals with stage III COPD. However, because only seven patients had stage IV COPD, the difference of NT-proBNP between stage III and stage IV did not reach statistical significance most likely because of a lack of statistical power, not because of an actual lack of difference. In fact, Fig. 1a shows a linear trend and Fig. 2a shows that for FEV1% below about 50%, the variability around the fitted regression line is quite high. This result was also confirmed when factors related to age and gender were eliminated because it is known that plasma NT-proBNP levels are affected by these factors. Second, plasma NT-proBNP levels significantly correlated with chronic respiratory failure identified as decreased PaO2 and increased PaCO2. Third, plasma NT-proBNP levels were significantly associated with systolic pulmonary artery pressure.
The natriuretic peptide family consists of three peptides: atrial natriuretic peptide (ANP), BNP, and C-type natriuretic peptide (CNP). These neurohormones are released in response to hemodynamic stress and help regulate intravascular volume homeostasis. Circulating ANP and BNP levels are elevated several-fold in patients with cor pulmonale, even in the absence of impaired left ventricular function [20]. Presumably, right atrial distention or overactivity in response to increased right ventricular afterload stimulates their release. Additionally, BNP is the predominant natriuretic peptide that modulates responses of the pulmonary vasculature to hypoxia [21]. BNP may play an important role in attenuating the pressor response that is likely to occur in humans in the presence of alveolar hypoxia.
The mechanism underlying high plasma NT-proBNP levels in patients with stable COPD is not well understood. There have been few studies that examined plasma NT-proBNP levels in patients with stable COPD. A recent study demonstrated that the overall diagnostic utility of both NT-proBNP and BNP is lower for detecting heart failure in patients with chronic dyspnea and COPD than in individuals with acute dyspnea presenting at the emergency department [22]. Stolz et al. [23] showed that plasma BNP levels are significantly elevated during acute exacerbation of COPD compared to recovery. They hypothesized that the elevation of BNP levels is at least partly due to hypoxia-mediated contraction of small pulmonary arterioles, resulting in increased pulmonary arterial pressure and subsequent cardiac stress. However, this group failed to show a significant correlation between plasma BNP levels and oxygen saturation, PaO2, PaCO2, or FEV1 [23].
In the present study, we observed significant correlation between plasma NT-proBNP levels and %FEV1, PaO2, PaCO2, and systolic pulmonary artery pressure in patients with stable COPD. This result can be explained by the fact that decreased FEV1 may lead to increased air-trapping and hyperinflation of the lung. Such hyperinflation is associated with decreased cardiac function and may result in an increase of plasma NT-proBNP. Another explanation might be related to hypoxia or hypercapnia in patients with COPD. Chronic hypoxia induced by the progression of COPD could result in contraction of the small pulmonary arterioles, resulting in increased pulmonary arterial systolic pressure and increased plasma NT-proBNP levels.
In conclusion, we demonstrated that plasma NT-proBNP levels significantly increase with COPD severity, progression of chronic respiratory failure, and the presence of secondary pulmonary hypertension in patients with stable COPD. Our results suggest that plasma NT-proBNP can be a useful prognostic marker of COPD progression. This factor may also help identify cases of secondary pulmonary hypertension in patients with stable COPD.
References
Sudoh T, Minamino N, Kangawa K, Matsuo H (1998) Brain natriuretic peptide-32: N-terminal six amino acid extended form of brain natriuretic peptide identified in porcine brain. Biochem Biophys Res Commun 155(2):726–732
Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA, INSPIRE Investigators (2008) The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med 177(1):19–26
Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J, TORCH Investigators (2007) Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 356(8):775–789
Oswald-Mammosser M, Weitzenblum E, Quoix E, Moser G, Chaouat A, Charpentier C, Kessler R (1995) Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest 107(5):1193–1198
Nocturnal Oxygen Therapy Trial Group (1980) Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 93(3):391–398
Cowie MR, Struthers AD, Wood DA, Coats AJ, Thompson SG, Poole-Wilson PA, Sutton GC (1997) Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet 350(9088):1349–1353
Landray MJ, Lehman R, Arnold I (2000) Measuring brain natriuretic peptide in suspected left ventricular systolic dysfunction in general practice: cross-sectional study. BMJ 320(7240):985–986
Mekontso Dessap A, Leon R, Habibi A, Nzouakou R, Roudot-Thoraval F, Adnot S, Godeau B, Galacteros F, Brun-Buisson C, Brochard L, Maitre B (2008) Pulmonary hypertension and cor pulmonale during severe acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med 177(6):646–653
Leuchte HH, El Nounou M, Tuerpe JC, Hartmann B, Baumgartner RA, Vogeser M, Muehling O, Behr J (2007) N-terminal pro-brain natriuretic peptide and renal insufficiency as predictors of mortality in pulmonary hypertension. Chest 131(2):402–409
Ishii J, Nomura M, Ito M, Naruse H, Mori Y, Wang JH, Ishikawa T, Kurokawa H, Kondo T, Nagamura Y, Ezaki K, Watanabe Y, Hishida H (2000) Plasma concentration of brain natriuretic peptide as a biochemical marker for the evaluation of right ventricular overload and mortality in chronic respiratory disease. Clin Chim Acta 301(1–2):19–30
Bando M, Ishii Y, Sugiyama Y, Kitamura S (1999) Elevated plasma brain natriuretic peptide levels in chronic respiratory failure with cor pulmonale. Respir Med 93(7):507–514
Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J, Global Initiative for Chronic Obstructive Lung Disease (2007) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 176(6):532–555
Loke I, Squire IB, Davies JE, Ng L (2003) Reference ranges for natriuretic peptides for diagnostic use are dependent on age, gender and heart rate. Eur J Heart Fail 5(5):599–606
Krauser DG, Lloyd-Jones DM, Chae CU, Cameron R, Anwaruddin S, Baggish AL, Chen A, Tung R, Januzzi JL Jr (2005) Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. Am Heart J 149(4):744–750
Burke MA, Cotts WG (2007) Interpretation of B-type natriuretic peptide in cardiac disease and other comorbid conditions. Heart Fail Rev 12(1):23–36
Schena M, Clini E, Errera D, Quadri A (1996) Echo-Doppler evaluation of left ventricular impairment in chronic cor pulmonale. Chest 109(6):1446–1451
Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I et al (1989) Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 2(5):358–367
Garcia MJ, Thomas JD, Klein AL (1998) New Doppler echocardiographic applications for the study of diastolic function. J Am Coll Cardiol 32(4):865–875
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration (2006) Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145(4):247–254
Lang CC, Coutie WJ, Struthers AD, Dhillon DP, Winter JH, Lipworth BJ (1992) Elevated levels of brain natriuretic peptide in acute hypoxaemic chronic obstructive pulmonary disease. Clin Sci (Lond) 83(5):529–533
Aronson D, Burger AJ (2002) Intravenous nesiritide (human B-type natriuretic peptide) reduces plasma endothelin-1 levels in patients with decompensated congestive heart failure. Am J Cardiol 90(4):435–438
Rutten FH, Cramer MJ, Zuithoff NP, Lammers JW, Verweij W, Grobbee DE, Hoes AW (2007) Comparison of B-type natriuretic peptide assays for identifying heart failure in stable elderly patients with a clinical diagnosis of chronic obstructive pulmonary disease. Eur J Heart Fail 9(6–7):651–659
Stolz D, Breidthardt T, Christ-Crain M, Bingisser R, Miedinger D, Leuppi J, Mueller B, Tamm M, Mueller C (2008) Use of B-type natriuretic peptide in the risk stratification of acute exacerbations of COPD. Chest 133(5):1088–1094
Acknowledgments
This work was supported by a Chonnam National University Hospital Research Institute of Clinical Medicine grant (CRI09047-1).
Conflict of interest
The authors have no conflicts of interest to disclose.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Chi, S.Y., Kim, E.Y., Ban, H.J. et al. Plasma N-terminal Pro-brain Natriuretic Peptide: A Prognostic Marker in Patients with Chronic Obstructive Pulmonary Disease. Lung 190, 271–276 (2012). https://doi.org/10.1007/s00408-011-9363-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-011-9363-7