Abstract
Background
The purpose of this study was to assess the efficacy and toxicity of vandetanib in the second-line treatment for advanced non-small cell lung cancer (NSCLC).
Methods
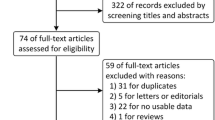
We systematically searched for randomized clinical trials that compared therapy with vandetanib versus standard second-line treatment, including docetaxel, pemetrexed, erlotinib, or gefitinib, as second-line treatment for patients with histologically proven non-small-cell lung cancer. The primary endpoint was overall survival (OS). Secondary endpoints were progression-free survival, overall response rate, and grade 3 or 4 toxicity. Data were extracted from the studies by two independent reviewers. The meta-analysis was performed by Stata version 10.0 software (Stata Corporation, College Station, TX, USA).
Results
Four randomized clinical trials (N = 3,292 patients) were eligible. Meta-analysis showed that there was significant improvement in PFS (hazards ration (HR), 0.91; 95% confidence interval (CI), 0.83−1.00; P = 0.039) and overall response rate (relative risk (RR), 1.49; 95% CI, 1.04−2.14; P = 0.03) in therapy with vandetanib group compared with standard second-line therapy group, although the pooled HR for overall survival (HR, 0.95; 95% CI, 0.88−1.03; P = 0.191) showed no significant difference between the two groups. In addition, there were less incidences of grade 3 or 4 anemia (RR, 0.39; 95% CI, 0.22−0.67; P = 0.001) in therapy with vandetanib group. With regard to the risk of grade 3 or 4 neutropenia (RR, 1.19; 95% CI, 1.0–1.43; P = 0.054), diarrhea (RR, 1.38; 95% CI, 1.0−1.94; P = 0.059), nausea and vomiting (RR, 0.77; 95% CI, 0.48−1.26; P = 0.308), rash (RR, 2.83; 95% CI, 0.73−10.9; P = 0.131), cough (RR, 1.19; 95% CI, 1.0−1.43; P = 0.054), and fatigue (RR, 1.0; 95% CI, 0.747−1.35; P = 0.971), there was no significant difference between the two groups.
Conclusions
Therapy with vandetanib offered a clinically meaningful and statistically significant improvement in PFS and ORR in patients with advanced NSCLC but did not benefit overall survival. Therapy with vandetanib regimens might be suggested as second-line treatment for advanced NSCLC based on a similar toxicity profile compared with standard second-line therapy.





Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ (2008) Cancer statistics, 2008. CA Cancer J Clin 58:71–96
Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24:2137–2150
Felip E, Rosell R, Pampaloni G (2008) Pemetrexed as second-line therapy for advanced non-small-cell lung cancer (NSCLC). Ther Clin Risk Manag 4:579–585
Russo FBA, Pampaloni G (2008) Pemetrexed single agent chemotherapy in previously treated patients with local advanced or metastatic non small cell lung cancer. BMC Cancer 8:216–223
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabárbara P, Seymour L, National Cancer Institute of Canada Clinical Trials Group (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353:123–132
Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, Li LY, Watkins CL, Sellers MV, Lowe ES, Sun Y, Liao ML, Osterlind K, Reck M, Armour AA, Shepherd FA, Lippman SM, Douillard JY (2008) Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet 372:1809–1818
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D, Eek R, Horai T, Noda K, Takata I, Smit E, Averbuch S, Macleod A, Feyereislova A, Dong RP, Baselga J (2003) Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small- cell lung cancer. J Clin Oncol 21:2237–2246
Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, Thongprasert S, Tan EH, Pemberton K, Archer V, Carroll K (2005) Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomized, placebo-controlled, multi-centre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366:1527–1537
Herbst RS, Heymach JV, O’Reilly MS, Onn A, Ryan AJ (2007) Vandetanib (ZD6474): an orally available receptor tyrosine kinase inhibitor that selectively targets pathways critical for tumor growth and angiogenesis. Expert Opin Investig Drugs 16:239–249
Wedge SR, Ogilvie DJ, Dukes M, Kendrew J, Chester R, Jackson JA, Boffey SJ, Valentine PJ, Curwen JO, Musgrove HL, Graham GA, Hughes GD, Thomas AP, Stokes ES, Curry B, Richmond GH, Wadsworth PF, Bigley AL, Hennequin LF (2002) ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res 62:4645–4655
Carlomagno F, Vitagliano D, Guida T, Ciardiello F, Tortora G, Vecchio G, Ryan AJ, Fontanini G, Fusco A, Santoro M (2002) ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res 62:7284–7290
Beaudry P, Nilsson M, Rioth M, Prox D, Poon D, Xu L, Zweidler-Mckay P, Ryan A, Folkman J, Ryeom S, Heymach J (2008) Potent anti-tumor effects of ZD6474 on neuroblastoma via dual targeting of tumor cells and tumor endothelium. Mol Cancer Ther 7:418–424
Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP (1998) Does quality of reports of randomized trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 352:609–613
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17:2815–2834
Zintzaras E, Ioannidis JP (2005) Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol 28:123–137
Yusuf S, Peto R, Jones DR, Collins R, Sleight P (1985) Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 27:335–371
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Herbst RS, Sun Y, Eberhardt WE, Germonpré P, Saijo N, Zhou C, Wang J, Li L, Kabbinavar F, Ichinose Y, Qin S, Zhang L, Biesma B, Heymach JV, Langmuir P, Kennedy SJ, Tada H, Johnson BE (2010) Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol 11:619–626
Natale RB, Thongprasert S, Greco FA, Thomas M, Tsai CM, Sunpaweravong P, Ferry D, Mulatero C, Whorf R, Thompson J, Barlesi F, Langmuir P, Gogov S, Rowbottom JA, Goss GD (2011) Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 29:1059–1066
de Boer RH, Arrieta Ó, Yang CH, Gottfried M, Chan V, Raats J, de Marinis F, Abratt RP, Wolf J, Blackhall FH, Langmuir P, Milenkova T, Read J, Vansteenkiste JF (2011) Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol 29:1067–1074
Heymach JV, Johnson BE, Prager D, Csada E, Roubec J, Pesek M, Spásová I, Belani CP, Bodrogi I, Gadgeel S, Kennedy SJ, Hou J, Herbst RS (2007) Randomized, placebo-controlled phase II study of vandetanib plus docetaxel in previously treated non small-cell lung cancer. J Clin Oncol 25:4270–4277
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qi, WX., Tang, LN., He, AN. et al. The Role of Vandetanib in the Second-Line Treatment for Advanced Non-Small-Cell-Lung Cancer: A Meta-Analysis of Four Randomized Controlled Trials. Lung 189, 437–443 (2011). https://doi.org/10.1007/s00408-011-9332-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-011-9332-1