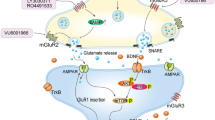
Abstract
Triggered by the ground-breaking finding that ketamine exerts robust and rapid-acting antidepressant effects in patients with treatment-resistant depression, glutamatergic systems have attracted attention as targets for the development of novel antidepressants. Among glutamatergic systems, group II metabotropic glutamate (mGlu) receptors, consisting of mGlu2 and mGlu3 receptors, are of interest because of their modulatory roles in glutamatergic transmission. Accumulating evidence has indicated that mGlu2/3 receptor antagonists have antidepressant-like effects in rodent models that mirror those of ketamine and that mGlu2/3 receptor antagonists also share underlying mechanisms with ketamine that are responsible for these antidepressant-like actions. Importantly, contrary to their antidepressant-like profile, preclinical studies have revealed that mGlu2/3 receptor antagonists are devoid of ketamine-like adverse effects, such as psychotomimetic-like behavior, abuse potential and neurotoxicity. Despite some discouraging results for an mGlu2/3 receptor antagonist decoglurant (classified as a negative allosteric modulator [NAM]) in patients with major depressive disorder, clinical trials of two mGlu2/3 receptor antagonists, a phase 2 trial of TS-161 (an orthosteric antagonist) and a phase 1 trial of DSP-3456 (a NAM), are presently on-going. mGlu2/3 receptors still hold promise for the development of safer and more efficacious antidepressants.


Similar content being viewed by others
Data availability
This article does not contain any original data.
References
Gerhard DM, Wohleb ES, Duman RS (2016) Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today 21:454–464. https://doi.org/10.1016/j.drudis.2016.01.016
Skolnick P, Popik P, Trullas R (2009) Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci 30:563–569. https://doi.org/10.1016/j.tips.2009.09.002
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. https://doi.org/10.1016/s0006-3223(99)00230-9
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. https://doi.org/10.1001/archpsyc.63.8.856
Henter ID, Park LT, Zarate CA Jr (2021) Novel glutamatergic modulators for the treatment of mood disorders: current status. CNS Drugs 35:527–543. https://doi.org/10.1007/s40263-021-00816-x
Leal GC, Bandeira ID, Correia-Melo FS, Telles M, Mello RP, Vieira F, Lima CS, Jesus-Nunes AP, Guerreiro-Costa LNF, Marback RF, Caliman-Fontes AT, Marques BLS, Bezerra MLO, Dias-Neto AL, Silva SS, Sampaio AS, Sanacora G, Turecki G, Loo C, Lacerda ALT, Quarantini LC (2021) Intravenous arketamine for treatment-resistant depression: open-label pilot study. Eur Arch Psychiatry Clin Neurosci 271:577–582. https://doi.org/10.1007/s00406-020-01110-5
Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K (2015) R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5:e632. https://doi.org/10.1038/tp.2015.136
Conn PJ, Pin JP (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37:205–237. https://doi.org/10.1146/annurev.pharmtox.37.1.205
Schoepp DD, Conn PJ (1993) Metabotropic glutamate receptors in brain function and pathology. Trends Pharmacol Sci 14:13–20. https://doi.org/10.1016/0165-6147(93)90107-u
Schaffhauser H, Richards JG, Cartmell J, Chaboz S, Kemp JA, Klingelschmidt A, Messer J, Stadler H, Woltering T, Mutel V (1998) In vitro binding characteristics of a new selective group II metabotropic glutamate receptor radioligand, [3H]LY354740, in rat brain. Mol Pharmacol 53:228–233
Moghaddam B, Javitt D (2012) From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 37:4–15. https://doi.org/10.1038/npp.2011.181
Sanacora G, Treccani G, Popoli M (2012) Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62:63–77. https://doi.org/10.1016/j.neuropharm.2011.07.036
Palucha A, Pilc A (2007) Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol Ther 115:116–147. https://doi.org/10.1016/j.pharmthera.2007.04.007
Kenny PJ, Markou A (2004) The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci 25:265–272. https://doi.org/10.1016/j.tips.2004.03.009
Kinon BJ, Millen BA, Zhang L, McKinzie DL (2015) Exploratory analysis for a targeted patient population responsive to the metabotropic glutamate 2/3 receptor agonist pomaglumetad methionil in schizophrenia. Biol Psychiatry 78:754–762. https://doi.org/10.1016/j.biopsych.2015.03.016
Dunayevich E, Erickson J, Levine L, Landbloom R, Schoepp DD, Tollefson GD (2008) Efficacy and tolerability of an mGlu2/3 agonist in the treatment of generalized anxiety disorder. Neuropsychopharmacology 33:1603–1610. https://doi.org/10.1038/sj.npp.1301531
Jing XY, Wang Y, Zou HW, Li ZL, Liu YJ, Li LF (2021) mGlu2/3 receptor in the prelimbic cortex is implicated in stress resilience and vulnerability in mice. Eur J Pharmacol 906:174231. https://doi.org/10.1016/j.ejphar.2021.174231
Kawasaki T, Ago Y, Yano K, Araki R, Washida Y, Onoe H, Chaki S, Nakazato A, Hashimoto H, Baba A, Takuma K, Matsuda T (2011) Increased binding of cortical and hippocampal group II metabotropic glutamate receptors in isolation-reared mice. Neuropharmacology 60:397–404. https://doi.org/10.1016/j.neuropharm.2010.10.009
Feyissa AM, Woolverton WL, Miguel-Hidalgo JJ, Wang Z, Kyle PB, Hasler G, Stockmeier CA, Iyo AH, Karolewicz B (2010) Elevated level of metabotropic glutamate receptor 2/3 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry 34:279–283. https://doi.org/10.1016/j.pnpbp.2009.11.018
McOmish CE, Pavey G, Gibbons A, Hopper S, Udawela M, Scarr E, Dean B (2016) Lower [3H]LY341495 binding to mGlu2/3 receptors in the anterior cingulate of subjects with major depressive disorder but not bipolar disorder or schizophrenia. J Affect Disord 190:241–248. https://doi.org/10.1016/j.jad.2015.10.004
Nasca C, Xenos D, Barone Y, Caruso A, Scaccianoce S, Matrisciano F, Battaglia G, Mathé AA, Pittaluga A, Lionetto L, Simmaco M, Nicoletti F (2013) L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc Natl Acad Sci USA 110:4804–4809. https://doi.org/10.1073/pnas.1216100110
Wierońska JM, Legutko B, Dudys D, Pilc A (2008) Olfactory bulbectomy and amitriptyline treatment influences mGlu receptors expression in the mouse brain hippocampus. Pharmacol Rep 60:844–855
Chaki S, Yoshikawa R, Hirota S, Shimazaki T, Maeda M, Kawashima N, Yoshimizu T, Yasuhara A, Sakagami K, Okuyama S, Nakanishi S, Nakazato A (2014) MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology 46:457–467. https://doi.org/10.1016/j.neuropharm.2003.10.009
Witkin JM, Ornstein PL, Mitch CH, Li R, Smith SC, Heinz BA, Wang XS, Xiang C, Carter JH, Anderson WH, Li X, Broad LM, Pasqui F, Fitzjohn SM, Sanger HE, Smith JL, Catlow J, Swanson S, Monn JA (2017) In vitro pharmacological and rat pharmacokinetic characterization of LY3020371, a potent and selective mGlu2/3 receptor antagonist. Neuropharmacology 115:100–114. https://doi.org/10.1016/j.neuropharm.2015.12.021
Campo B, Kalinichev M, Lambeng N, El Yacoubi M, Royer-Urios I, Schneider M, Legrand C, Parron D, Girard F, Bessif A, Poli S, Vaugeois JM, Le Poul E, Celanire S (2011) Characterization of an mGluR2/3 negative allosteric modulator in rodent models of depression. J Neurogenet 25:152–166. https://doi.org/10.3109/01677063.2011.627485
Lavreysen H, Langlois X, Ahnaou A, Drinkenburg W, te Riele P, Biesmans I, Van der Linden I, Peeters L, Megens A, Wintmolders C, Cid JM, Trabanco AA, Andrés JI, Dautzenberg FM, Lütjens R, Macdonald G, Atack JR (2013) Pharmacological characterization of JNJ-40068782, a new potent, selective, and systemically active positive allosteric modulator of the mGlu2 receptor and its radioligand [3H]JNJ-40068782. J Pharmacol Exp Ther 346:514–527. https://doi.org/10.1124/jpet.113.204990
Chaki S (2017) mGlu2/3 receptor antagonists as novel antidepressants. Trends Pharmacol Sci 38:569–580. https://doi.org/10.1016/j.tips.2017.03.008
Witkin JM (2020) mGlu2/3 receptor antagonism: A mechanism to induce rapid antidepressant effects without ketamine-associated side-effects. Pharmacol Biochem Behav 190:172854. https://doi.org/10.1016/j.pbb.2020.172854
Dong C, Zhang JC, Yao W, Ren Q, Ma M, Yang C, Chaki S, Hashimoto K (2017) Rapid and sustained antidepressant action of the mGlu2/3 receptor antagonist MGS0039 in the social defeat stress model: comparison with ketamine. Int J Neuropsychopharmacol 20:228–236. https://doi.org/10.1093/ijnp/pyw089
Dong C, Tian Z, Fujita Y, Fujita A, Hino N, Iijima M, Hashimoto K (2022) Antidepressant-like actions of the mGlu2/3 receptor antagonist TP0178894 in the chronic social defeat stress model: comparison with escitalopram. Pharmacol Biochem Behav 212:173316. https://doi.org/10.1016/j.pbb.2021.173316
Dwyer JM, Lepack AE, Duman RS (2013) mGluR2/3 blockade produces rapid and long-lasting reversal of anhedonia caused by chronic stress exposure. J Mol Psychiatry 1:15. https://doi.org/10.1186/2049-9256-1-15.eCollection2013
Pałucha-Poniewiera A, Podkowa K, Rafało-Ulińska A (2021) The group II mGlu receptor antagonist LY341495 induces a rapid antidepressant-like effect and enhances the effect of ketamine in the chronic unpredictable mild stress model of depression in C57BL/6J mice. Prog Neuropsychopharmacol Biol Psychiatry 109:110239. https://doi.org/10.1016/j.pnpbp.2020.110239
Seo MK, Lee JA, Jeong S, Seog DH, Lee JG, Park SW (2022) Effects of chronic LY341495 on hippocampal mTORC1 signaling in mice with chronic unpredictable stress-induced depression. Int J Mol Sci 23:6416. https://doi.org/10.3390/ijms23126416
Koike H, Iijima M, Chaki S (2013) Effects of ketamine and LY341495 on the depressive-like behavior of repeated corticosterone-injected rats. Pharmacol Biochem Behav 107:20–23. https://doi.org/10.1016/j.pbb.2013.03.017
Highland JN, Zanos P, Georgiou P, Gould TD (2019) Group II metabotropic glutamate receptor blockade promotes stress resilience in mice. Neuropsychopharmacology 44:1788–1796. https://doi.org/10.1038/s41386-019-0380-1
Witkin JM, Monn JA, Li J, Johnson B, McKinzie DL, Wang XS, Heinz BA, Li R, Ornstein PL, Smith SC, Mitch CH, Calligaro DO, Swanson S, Allen D, Phillips K, Gilmour G (2017) Preclinical predictors that the orthosteric mGlu2/3 receptor antagonist LY3020371 will not engender ketamine-associated neurotoxic, motor, cognitive, subjective, or abuse-liability-related effects. Pharmacol Biochem Behav 155:43–55. https://doi.org/10.1016/j.pbb.2017.03.001
Goeldner C, Ballard TM, Knoflach F, Wichmann J, Gatti S, Umbricht D (2013) Cognitive impairment in major depression and the mGlu2 receptor as a therapeutic target. Neuropharmacology 64:337–346. https://doi.org/10.1016/j.neuropharm.2012.08.001
Shimazaki T, Kaku A, Chaki S (2007) Blockade of the metabotropic glutamate 2/3 receptors enhances social memory via the AMPA receptor in rats. Eur J Pharmacol 575:94–97. https://doi.org/10.1016/j.ejphar.2007.08.006
Podkowa K, Pochwat B, Brański P, Pilc A, Pałucha-Poniewiera A (2016) Group II mGlu receptor antagonist LY341495 enhances the antidepressant-like effects of ketamine in the forced swim test in rats. Psychopharmacology 233:2901–2914. https://doi.org/10.1007/s00213-016-4325-7
Zanos P, Highland JN, Stewart BW, Georgiou P, Jenne CE, Lovett J, Morris PJ, Thomas CJ, Moaddel R, Zarate CA Jr, Gould TD (2019) (2R,6R)-hydroxynorketamine exerts mGlu 2 receptor-dependent antidepressant actions. Proc Natl Acad Sci USA 116:6441–6450. https://doi.org/10.1073/pnas.1819540116
Podkowa K, Podkowa A, Sałat K, Lenda T, Pilc A, Pałucha-Poniewiera A (2016) Antidepressant-like effects of scopolamine in mice are enhanced by the group II mGlu receptor antagonist LY341495. Neuropharmacology 111:169–179. https://doi.org/10.1016/j.neuropharm.2016.08.031
Drevets WC, Zarate CA Jr, Furey ML (2013) Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psychiatry 73:1156–1163. https://doi.org/10.1016/j.biopsych.2012.09.031
Rafało-Ulińska A, Brański P, Pałucha-Poniewiera A (2022) Combined administration of (R)-ketamine and the mGlu2/3 receptor antagonist LY341495 induces rapid and sustained effects in the CUMS model of depression via a TrkB/BDNF-dependent mechanism. Pharmaceuticals (Basel) 15:125. https://doi.org/10.3390/ph15020125
Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi JI, Hashimoto K, Chaki S (2017) Antidepressant potential of (R)-ketamine in rodent models: comparison with (S)-ketamine. J Pharmacol Exp Ther 361:9–16. https://doi.org/10.1124/jpet.116.239228
Zhang JC, Li SX, Hashimoto K (2014) R (-)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav 116:137–141. https://doi.org/10.1016/j.pbb.2013.11.033
Nasca C, Bigio B, Zelli D, Nicoletti F, McEwen BS (2015) Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol Psychiatry 20:755–763. https://doi.org/10.1038/mp.2014.96
Fell MJ, Witkin JM, Falcone JF, Katner JS, Perry KW, Hart J, Rorick-Kehn L, Overshiner CD, Rasmussen K, Chaney SF, Benvenga MJ, Li X, Marlow DL, Thompson LK, Luecke SK, Wafford KA, Seidel WF, Edgar DM, Quets AT, Felder CC, Wang X, Heinz BA, Nikolayev A, Kuo MS, Mayhugh D, Khilevich A, Zhang D, Ebert PJ, Eckstein JA, Ackermann BL, Swanson SP, Catlow JT, Dean RA, Jackson K, Tauscher-Wisniewski S, Marek GJ, Schkeryantz JM, Svensson KA (2011) N-(4-((2-(trifluoromethyl)-3-hydroxy-4-(isobutyryl)phenoxy)methyl)benzyl)-1-methyl-1H-imidazole-4-carboxamide (THIIC), a novel metabotropic glutamate 2 potentiator with potential anxiolytic/antidepressant properties: in vivo profiling suggests a link between behavioral and central nervous system neurochemical changes. J Pharmacol Exp Ther 336:165–177. https://doi.org/10.1124/jpet.110.172957
Krystal JH, Sanacora G, Duman RS (2013) Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry 73:1133–1141. https://doi.org/10.1016/j.biopsych.2013.03.026
Koike H, Iijima M, Chaki S (2011) Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology 61:1419–1423. https://doi.org/10.1016/j.neuropharm.2011.08.034
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329(5994):959–964. https://doi.org/10.1126/science.1190287
Lepack AE, Bang E, Lee B, Dwyer JM, Duman RS (2016) Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology 111:242–252. https://doi.org/10.1016/j.neuropharm.2016.09.011
Yang C, Yang J, Luo A, Hashimoto K (2019) Molecular and cellular mechanisms underlying the antidepressant effects of ketamine enantiomers and its metabolites. Transl Psychiatry 9:280. https://doi.org/10.1038/s41398-019-0624-1
Zanos P, Gould TD (2018) Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 23:801–811. https://doi.org/10.1038/mp.2017.255
Alt A, Nisenbaum ES, Bleakman D, Witkin JM (2006) A role for AMPA receptors in mood disorders. Biochem Pharmacol 71:1273–1288. https://doi.org/10.1016/j.bcp.2005.12.022
Hara H, Suzuki A, Kunugi A, Tajima Y, Yamada R, Kimura H (2021) TAK-653, an AMPA receptor potentiator with minimal agonistic activity, produces an antidepressant-like effect with a favorable safety profile in rats. Pharmacol Biochem Behav 211:173289. https://doi.org/10.1016/j.pbb.2021.173289
Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS (2002) Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 22:3251–3261. https://doi.org/10.1523/JNEUROSCI.22-08-03251.2002
Legutko B, Li X, Skolnick P (2001) Regulation of BDNF expression in primary neuron culture by LY392098, a novel AMPA receptor potentiator. Neuropharmacology 40:1019–1027. https://doi.org/10.1016/s0028-3908(01)00006-5
Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS (2011) Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761. https://doi.org/10.1016/j.biopsych.2010.12.015
Tang J, Xue W, Xia B, Ren L, Tao W, Chen C, Zhang H, Wu R, Wang Q, Wu H, Duan J, Chen G (2015) Involvement of normalized NMDA receptor and mTOR-related signaling in rapid antidepressant effects of Yueju and ketamine on chronically stressed mice. Sci Rep 5:13573. https://doi.org/10.1038/srep13573
Garro-Martínez E, Fullana MN, Florensa-Zanuy E, Senserrich J, Paz V, Ruiz-Bronchal E, Adell A, Castro E, Díaz Á, Pazos Á, Bortolozzi A, Pilar-Cuéllar F (2021) mTOR knockdown in the infralimbic cortex evokes a depressive-like state in mouse. Int J Mol Sci 22:8671. https://doi.org/10.3390/ijms22168671
Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, Karolewicz B (2011) The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 35:1774–1779. https://doi.org/10.1016/j.pnpbp.2011.05.010
Salort G, Hernández-Hernández E, García-Fuster MJ, García-Sevilla JA (2020) Regulation of cannabinoid CB 1 and CB 2 receptors, neuroprotective mTOR and pro-apoptotic JNK1/2 kinases in postmortem prefrontal cortex of subjects with major depressive disorder. J Affect Disord 276:626–635. https://doi.org/10.1016/j.jad.2020.07.074
Abdallah CG, Averill LA, Gueorguieva R, Goktas S, Purohit P, Ranganathan M, Sherif M, Ahn KH, D’Souza DC, Formica R, Southwick SM, Duman RS, Sanacora G, Krystal JH (2020) Modulation of the antidepressant effects of ketamine by the mTORC1 inhibitor rapamycin. Neuropsychopharmacology 45:990–997. https://doi.org/10.1038/s41386-020-0644-9
Witkin JM, Monn JA, Schoepp DD, Li X, Overshiner C, Mitchell SN, Carter G, Johnson B, Rasmussen K, Rorick-Kehn LM (2016) The rapidly acting antidepressant ketamine and the mGlu2/3 receptor antagonist LY341495 rapidly engage dopaminergic mood circuits. J Pharmacol Exp Ther 358:71–82. https://doi.org/10.1124/jpet.116.233627
Karasawa J, Yoshimizu T, Chaki S (2006) A metabotropic glutamate 2/3 receptor antagonist, MGS0039, increases extracellular dopamine levels in the nucleus accumbens shell. Neurosci Lett 393:127–130. https://doi.org/10.1016/j.neulet.2005.09.058
Karasawa J, Kotani M, Kambe D, Chaki S (2010) AMPA receptor mediates mGlu 2/3 receptor antagonist-induced dopamine release in the rat nucleus accumbens shell. Neurochem Int 57:615–619. https://doi.org/10.1016/j.neuint.2010.07.011
Iijima M, Koike H, Chaki S (2013) Effect of an mGlu2/3 receptor antagonist on depressive behavior induced by withdrawal from chronic treatment with methamphetamine. Behav Brain Res 246:24–28. https://doi.org/10.1016/j.bbr.2013.02.039
Karasawa J, Shimazaki T, Kawashima N, Chaki S (2005) AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res 1042:92–98. https://doi.org/10.1016/j.brainres.2005.02.032
Nishitani N, Nagayasu K, Asaoka N, Yamashiro M, Shirakawa H, Nakagawa T, Kaneko S (2014) Raphe AMPA receptors and nicotinic acetylcholine receptors mediate ketamine-induced serotonin release in the rat prefrontal cortex. Int J Neuropsychopharmacol 17:1321–1326. https://doi.org/10.1017/S1461145714000649
Pham TH, Mendez-David I, Defaix C, Guiard BP, Tritschler L, David DJ, Gardier AM (2017) Ketamine treatment involves medial prefrontal cortex serotonin to induce a rapid antidepressant-like activity in BALB/cJ mice. Neuropharmacology 112:198–209. https://doi.org/10.1016/j.neuropharm.2016.05.010
Fukumoto K, Iijima M, Chaki S (2016) The Antidepressant Effects of an mGlu2/3 Receptor Antagonist and Ketamine Require AMPA Receptor Stimulation in the mPFC and Subsequent Activation of the 5-HT Neurons in the DRN. Neuropsychopharmacology 41:1046–1056. https://doi.org/10.1038/npp.2015.233
Gasull-Camós J, Tarrés-Gatius M, Artigas F, Castañé A (2017) Glial GLT-1 blockade in infralimbic cortex as a new strategy to evoke rapid antidepressant-like effects in rats. Transl Psychiatry 7(2):e1038. https://doi.org/10.1038/tp.2017.7
Gasull-Camós J, Martínez-Torres S, Tarrés-Gatius M, Ozaita A, Artigas F, Castañé A (2018) Serotonergic mechanisms involved in antidepressant-like responses evoked by GLT-1 blockade in rat infralimbic cortex. Neuropharmacology 139:41–51. https://doi.org/10.1016/j.neuropharm.2018.06.029
Pham TH, Defaix C, Xu X, Deng SX, Fabresse N, Alvarez JC, Landry DW, Brachman RA, Denny CA, Gardier AM (2018) Common neurotransmission recruited in (R, S)-ketamine and (2R,6R)-hydroxynorketamine-induced sustained antidepressant-like effects. Biol Psychiatry 84:e3–e6. https://doi.org/10.1016/j.biopsych.2017.10.020
Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, Adhikari A, Tye KM, Frank LM, Deisseroth K (2012) A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature 492:428–432. https://doi.org/10.1038/nature11617
Fukumoto K, Iijima M, Funakoshi T, Chaki S (2018) 5-HT 1A receptor stimulation in the medial prefrontal cortex mediates the antidepressant effects of mGlu2/3 receptor antagonist in mice. Neuropharmacology 137:96–103. https://doi.org/10.1016/j.neuropharm.2018.05.001
Fukumoto K, Iijima M, Funakoshi T, Chaki S (2018) Role of 5-HT1A receptor stimulation in the medial prefrontal cortex in the sustained antidepressant effects of ketamine. Int J Neuropsychopharmacol 21:371–381. https://doi.org/10.1093/ijnp/pyx116
Fukumoto K, Fogaça MV, Liu RJ, Duman CH, Li XY, Chaki S, Duman RS (2020) Medial PFC AMPA receptor and BDNF signaling are required for the rapid and sustained antidepressant-like effects of 5-HT 1A receptor stimulation. Neuropsychopharmacology 45:1725–1734. https://doi.org/10.1038/s41386-020-0705-0
Witkin JM, Mitchell SN, Wafford KA, Carter G, Gilmour G, Li J, Eastwood BJ, Overshiner C, Li X, Rorick-Kehn L, Rasmussen K, Anderson WH, Nikolayev A, Tolstikov VV, Kuo MS, Catlow JT, Li R, Smith SC, Mitch CH, Ornstein PL, Swanson S, Monn JA (2017) Comparative effects of LY3020371, a potent and selective metabotropic glutamate (mGlu) 2/3 receptor antagonist, and ketamine, a noncompetitive N-methyl-d-aspartate receptor antagonist in rodents: Evidence supporting the use of mGlu2/3 antagonists, for the treatment of depression. J Pharmacol Exp Ther 361:68–86. https://doi.org/10.1124/jpet.116.238121
Gleason SD, Li X, Smith IA, Ephlin JD, Wang XS, Heinz BA, Carter JH, Baez M, Yu J, Bender DM, Witkin JM (2013) mGlu2/3 agonist-induced hyperthermia: an in vivo assay for detection of mGlu2/3 receptor antagonism and its relation to antidepressant-like efficacy in mice. CNS Neurol Disord Drug Targets 12:554–566. https://doi.org/10.2174/18715273113129990079
Engers JL, Bollinger KA, Weiner RL, Rodriguez AL, Long MF, Breiner MM, Chang S, Bollinger SR, Bubser M, Jones CK, Morrison RD, Bridges TM, Blobaum AL, Niswender CM, Conn PJ, Emmitte KA, Lindsley CW (2017) Design and synthesis of N-aryl phenoxyethoxy pyridinones as highly selective and CNS penetrant mGlu 3 NAMs. ACS Med Chem Lett 8:925–930. https://doi.org/10.1021/acsmedchemlett.7b00249.eCollection2017
Joffe ME, Santiago CI, Oliver KH, Maksymetz J, Harris NA, Engers JL, Lindsley CW, Winder DG, Conn PJ (2020) mGlu 2 and mGlu 3 negative allosteric modulators divergently enhance thalamocortical transmission and exert rapid antidepressant-like effects. Neuron 105:46-59.e3. https://doi.org/10.1016/j.neuron.2019.09.044
Witkin JM, Pandey KP, Smith JL (2022) Clinical investigations of compounds targeting metabotropic glutamate receptors. Pharmacol Biochem Behav 219:173446. https://doi.org/10.1016/j.pbb.2022.173446
Umbricht D, Niggli M, Sanwald-Ducray P, Deptula D, Moore R, Grünbauer W, Boak L, Fontoura P (2020) Randomized, double-blind, placebo-controlled trial of the mGlu2/3 negative allosteric modulator decoglurant in partially refractory major depressive disorder. J Clin Psychiatry 81:18m12470. https://doi.org/10.4088/JCP.18m12470
Sheffler DJ, Bicakci MB, Antwan A, Prakash N, Standard EM, Velicelebi G, Gadient RA, Hutchison JH, Panickar DR, Limpert AS, Cosford NDP, Der-Avakian A (2019) In vitro and in vivo characterization of group II mGlu receptor negative allosteric modulators as an alternative to ketamine for depression. Soc Neurosc Abst 684:20
Watanabe M, Marcy B, Hiroki A, Watase H, Kinoshita K, Iijima M, Marumo T, Zarate CA, Chaki S (2022) Evaluation of the safety, tolerability, and pharmacokinetic profiles of TP0473292 (TS-161), a prodrug of a novel orthosteric mGlu2/3 receptor antagonist TP0178894, in healthy subjects and its antidepressant-like effects in rodents. Int J Neuropsychopharmacol 25:106–117. https://doi.org/10.1093/ijnp/pyab062
Nakamura M, Kawakita Y, Yasuhara A, Fukasawa Y, Yoshida K, Sakagami K, Nakazato A (2006) In vitro and in vivo evaluation of the metabolism and bioavailability of ester prodrugs of mgs0039 (3-(3,4-dichlorobenzyloxy)-2-amino-6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic Acid), a potent metabotropic glutamate receptor antagonist. Drug Metab Dispos 34:369–374. https://doi.org/10.1124/dmd.105.006213
Yasuhara A, Nakamura M, Sakagami K, Shimazaki T, Yoshikawa R, Chaki S, Ohta H, Nakazato A (2006) Prodrugs of 3-(3,4-dichlorobenzyloxy)-2-amino-6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (MGS0039): a potent and orally active group II mGluR antagonist with antidepressant-like potential. Bioorg Med Chem 14:4193–4207. https://doi.org/10.1016/j.bmc.2006.01.060
Gardient RS, Wedel P, Frisbie V, Leuchter AF, Targum SD, Truong C, Hutchinson JH (2012) Safety, pharmacokinetic and pharmacodynamic profile of BCI-632, a selective metabotropic glutamate 2/3 receptor antagonist, in healthy human subjects. Soc Neurosci Abst 841:20
Development Pipeline (As of July 29, 2022) Sumitomo Pharma Co., Ltd. https://www.sumitomo-pharma.com/rd/clinical/pdf/epip20220729.pdf. Accessed Oct 19 2022.
Author information
Authors and Affiliations
Contributions
The first draft of the manuscript was written by Shigeyuki Chaki and Mai Watanabe. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Shigeyuki Chaki is a full-time employee of Taisho Pharmaceutical Co., Ltd., and Mai Watanabe is a full-time employee of Taisho Pharmaceutical R&D Inc.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chaki, S., Watanabe, M. mGlu2/3 receptor antagonists for depression: overview of underlying mechanisms and clinical development. Eur Arch Psychiatry Clin Neurosci 273, 1451–1462 (2023). https://doi.org/10.1007/s00406-023-01561-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-023-01561-6