Abstract
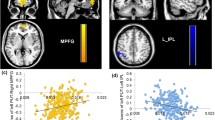
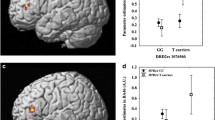
Research integrating molecular and imaging data provides important insights into how the genetic profile associated with dopamine signaling influences inter-individual differences in brain functions. However, the effects of genetic variations in dopamine signaling on the heterogeneity of brain changes induced by repetitive transcranial magnetic stimulation (rTMS) still remain unclear. The current study examined the composite effects of genetic variations in dopamine-related genes on rTMS-induced brain responses in terms of the functional network connectivity and working memory performance. Healthy individuals (n = 30) participated in a randomized, double-blind, sham-controlled study with a crossover design of five consecutive days where active rTMS or sham stimulation sessions were administered over the left dorsolateral prefrontal cortex (DLPFC) of the brain. Participants were mostly women (n = 29) and genotyped for polymorphisms in the catechol-O-methyltransferase and D2 dopamine receptor genes and categorized according to their genetic composite scores: high vs. low dopamine signaling groups. Pre- and post-intervention data of resting-state functional magnetic resonance imaging and working memory performance were obtained from 27 individuals with active rTMS and 30 with sham stimulation sessions. The mean functional connectivity within the resting-state networks centered on the DLPFC increased in the high dopamine signaling group. Working memory performance also improved with rTMS in the high dopamine signaling group compared to that in the low dopamine signaling group. The present results suggest that genetic predisposition to higher dopamine signaling may be a promising neurobiological predictor for rTMS effects on cognitive enhancement.
Trial registration: ClinicalTrials.gov (NCT02932085).




Similar content being viewed by others
References
Hoogendam JM, Ramakers GM, Di Lazzaro V (2010) Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul 3:95–118
Silverstein WK, Noda Y, Barr MS et al (2015) Neurobiological predictors of response to dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation in depression: a systematic review. Depress Anxiety 32:8718–8791
Ridding MC, Ziemann U (2010) Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol 588:2291–2304
Bocchio-Chiavetto L, Miniussi C, Zanardini R et al (2008) 5-HTTLPR and BDNF Val66Met polymorphisms and response to rTMS treatment in drug resistant depression. Neurosci Lett 437:130–134
Malaguti A, Rossini D, Lucca A et al (2011) Role of COMT, 5-HT(1A), and SERT genetic polymorphisms on antidepressant response to transcranial magnetic stimulation. Depress Anxiety 28:568–573
Zanardi R, Magri L, Rossini D et al (2007) Role of serotonergic gene polymorphisms on response to transcranial magnetic stimulation in depression. Eur Neuropsychopharmacol 17:651–657
Otani S, Blond O, Desce JM et al (1998) Dopamine facilitates long-term depression of glutamatergic transmission in rat prefrontal cortex. Neurosci 85:669–676
Cooke SF, Bliss TV (2006) Plasticity in the human central nervous system. Brain 129:1659–1673
Ghanavati E, Salehinejad MA, De Melo L et al (2022) NMDA receptor-related mechanisms of dopaminergic modulation of tDCS-induced neuroplasticity. Cereb Cortex (Published Online First). https://doi.org/10.1093/cercor/bhac028
Nitsche MA, Lampe C, Antal A et al (2006) Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex. Eur J Neurosci 23:1651–1657
Monte-Silva K, Kuo MF, Thirugnanasambandam N et al (2009) Dose-dependent inverted U-shaped effect of dopamine (D2-like) receptor activation on focal and nonfocal plasticity in humans. J Neurosci 29:6124–6131
Keck ME, Welt T, Müller MB et al (2002) Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neuropharmacology 43:101–109
Funamizu H, Ogiue-Ikeda M, Mukai H et al (2005) Acute repetitive transcranial magnetic stimulation reactivates dopaminergic system in lesion rats. Neurosci Lett 383:77–81
Zangen A, Hyodo K (2002) Transcranial magnetic stimulation induces increases in extracellular levels of dopamine and glutamate in the nucleus accumbens. NeuroReport 13:2401–2405
Kanno M, Matsumoto M, Togashi H et al (2004) Effects of acute repetitive transcranial magnetic stimulation on dopamine release in the rat dorsolateral striatum. J Neurol Sci 217:73–81
Strafella AP, Paus T, Barrett J et al (2001) Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci 21:RC157
Pogarell O, Koch W, Pöpperl G et al (2006) Striatal dopamine release after prefrontal repetitive transcranial magnetic stimulation in major depression: preliminary results of a dynamic [123I] IBZM SPECT study. J Psychiatr Res 40:307–314
Pogarell O, Koch W, Pöpperl G et al (2007) Acute prefrontal rTMS increases striatal dopamine to a similar degree as D-amphetamine. Psychiatry Res 156:251–255
Cho SS, Strafella AP (2009) rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS ONE 4:e6725
Backman L, Nyberg L (2013) Dopamine and training-related working-memory improvement. Neurosci Biobehav Rev 37:2209–2219
Backman L, Nyberg L, Lindenberger U et al (2006) The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev 30:791–807
Cohen JD, Braver TS, Brown JW (2002) Computational perspectives on dopamine function in prefrontal cortex. Curr Opin Neurobiol 12:223–229
Brehmer Y, Rieckmann A, Bellander M et al (2011) Neural correlates of training-related working-memory gains in old age. Neuroimage 58:1110–1120
Backman L, Nyberg L, Soveri A et al (2011) Effects of working-memory training on striatal dopamine release. Science 333:718
Schacht JP (2016) COMT val158met moderation of dopaminergic drug effects on cognitive function: a critical review. Pharmacogenomics J 16:430–438
Cools R, Arnsten AFT (2022) Neuromodulation of prefrontal cortex cognitive function in primates: the powerful roles of monoamines and acetylcholine. Neuropsychopharmacology 47:309–328
Esslinger C, Schüler N, Sauer C et al (2014) Induction and quantification of prefrontal cortical network plasticity using 5 Hz rTMS and fMRI. Hum Brain Mapp 35:140–151
Bagherzadeh Y, Khorrami A, Zarrindast MR et al (2016) Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex enhances working memory. Exp Brain Res 234:1807–1818
Barr MS, Farzan F, Rajji TK et al (2013) Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biol Psychiatry 73:510–517
Begemann MJ, Brand BA, Ćurčić-Blake B et al (2020) Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: a meta-analysis. Psychol Med 50:2465–2486
Fitzgerald PB, Brown TL, Marston NA et al (2003) Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trial. Arch Gen Psychiatry 60:1002–1008
Battelli L, Grossman ED, Plow EB (2017) Local immediate versus long-range delayed changes in functional connectivity following rTMS on the visual attention network. Brain Stimul 10:263–269
van der Werf YD, Sanz-Arigita EJ, Menning S et al (2010) Modulating spontaneous brain activity using repetitive transcranial magnetic stimulation. BMC Neurosci 11:145
Tik M, Hoffmann A, Sladky R et al (2017) Towards understanding rTMS mechanism of action: stimulation of the DLPFC causes network-specific increase in functional connectivity. Neuroimage 162:289–296
Beynel L, Powers JP, Appelbaum LG (2020) Effects of repetitive transcranial magnetic stimulation on resting-state connectivity: a systematic review. Neuroimage 211:116596
Plomin R, Haworth CM, Davis OS (2009) Common disorders are quantitative traits. Nat Rev Genet 10:872–878
Davis C, Loxton NJ (2013) Addictive behaviors and addiction-prone personality traits: associations with a dopamine multilocus genetic profile. Addict Behav 38:2306–2312
Nikolova YS, Ferrell RE, Manuck SB et al (2011) Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology 36:1940–1947
Dillon DG, Bogdan R, Fagerness J et al (2010) Variation in TREK1 gene linked to depression-resistant phenotype is associated with potentiated neural responses to rewards in humans. Hum Brain Mapp 31:210–221
Kohno M, Nurmi EL, Laughlin CP et al (2016) Functional genetic variation in dopamine signaling moderates prefrontal cortical activity during risky decision making. Neuropsychopharmacology 41:695–703
Ising M, Lucae S, Binder EB et al (2009) A genomewide association study points to multiple loci that predict antidepressant drug treatment outcome in depression. Arch Gen Psychiatry 66:966–975
Tunbridge EM, Narajos M, Harrison CH et al (2019) Which dopamine polymorphisms are functional? systematic review and meta-analysis of COMT, DAT, DBH, DDC, DRD1-5, MAOA, MAOB, TH, VMAT1, and VMAT2. Biol Psychiatry 86:608–620
Chen J, Lipska BK, Halim N et al (2004) Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 75:807–821
Noble EP, Blum K, Ritchie T et al (1991) Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry 48:648–654
McClintock SM, Reti IM, Carpenter LL et al (2018) Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry 79:16cs10905
Beam W, Borckardt JJ, Reeves ST et al (2009) An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul 2:50–54
Foerster BU, Tomasi D, Caparelli EC (2005) Magnetic field shift due to mechanical vibration in functional magnetic resonance imaging. Magn Reson Med 54:1261–1267
Salimi-Khorshidi G, Douaud G, Beckmann CF et al (2014) Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage 90:449–468
Jenkinson M, Bannister P, Brady M et al (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841
Smith SM, Fox PT, Miller KL et al (2009) Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A 106:13040–13045
Brozoski TJ, Brown RM, Rosvold HE et al (1979) Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 205:929–932
Goldman-Rakic PS, Muly EC 3rd, Williams GV (2000) D(1) receptors in prefrontal cells and circuits. Brain Res Rev 31:295–301
Cools R, D’Esposito M (2011) Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69:e113-125
Bilder RM, Volavka J, Lachman HM et al (2004) The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology 29:1943–1961
Zhao F, Zhang X, Qin W et al (2015) Network-dependent modulation of COMT and DRD2 polymorphisms in healthy young adults. Sci Rep 5:17996
Tunbridge EM, Farrell SM, Harrison PJ et al (2013) Catechol-O-methyltransferase (COMT) influences the connectivity of the prefrontal cortex at rest. Neuroimage 68:49–54
Sanna A, Fattore L, Badas P et al (2021) The hypodopaminergic state ten years after: transcranial magnetic stimulation as a tool to test the dopamine hypothesis of drug addiction. Curr Opin Pharmacol 56:61–67
Kimberg DY, D’Esposito M, Farah MJ (1997) Effects of bromocriptine on human subjects depend on working memory capacity. NeuroReport 8:3581–3585
Apud JA, Mattay V, Chen J et al (2007) Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology 32:1011–1020
Furman DJ, White RL 3rd, Naskolnakorn J et al (2020) Effects of dopaminergic drugs on cognitive control processes vary by genotype. J Cogn Neurosci 32:804–821
Farrell SM, Tunbridge EM, Braeutigam S et al (2012) COMT Val(158)Met genotype determines the direction of cognitive effects produced by catechol-O-methyltransferase inhibition. Biol Psychiatry 71:538–544
Schacht JP, Voronin KE, Randall PK et al (2018) Dopaminergic genetic variation influences aripiprazole effects on alcohol self-administration and the neural response to alcohol cues in a randomized trial. Neuropsychopharmacology 43:1247–1256
Kaneko H, Miura I, Kanno-Nozaki K et al (2018) COMT Val 108/158 Met polymorphism and treatment response to aripiprazole in patients with acute schizophrenia. Neuropsychiatr Dis Treat 14:1657–1663
Peitl V, Štefanović M, Orlović I et al (2021) Long acting aripiprazole influences cognitive functions in recent onset schizophrenia. Psychopharmacology 238:1563–1573
Blasi G, Selvaggi P, Fazio L et al (2015) Variation in dopamine D2 and serotonin 5-HT2A receptor genes is associated with working memory processing and response to treatment with antipsychotics. Neuropsychopharmacology 40:1600–1608
Pettorruso M, Di Giuda D, Martinotti G et al (2019) Dopaminergic and clinical correlates of high-frequency repetitive transcranial magnetic stimulation in gambling addiction: a SPECT case study. Addict Behav 93:246–249
Malik S, Jacobs M, Cho SS et al (2018) Deep TMS of the insula using the H-coil modulates dopamine release: a crossover [11C] PHNO-PET pilot trial in healthy humans. Brain Imaging Behav 12:1306–1317
Jääskeläinen SK, Lindholm P, Valmunen T et al (2014) Variation in the dopamine D2 receptor gene plays a key role in human pain and its modulation by transcranial magnetic stimulation. Pain 155:2180–2187
Lang N, Speck S, Harms J et al (2008) Dopaminergic potentiation of rTMS-induced motor cortex inhibition. Biol Psychiatry 63:231–233
Hsieh TH, Huang YZ, Rotenberg A et al (2015) Functional dopaminergic neurons in substantia nigra are required for transcranial magnetic stimulation-induced motor plasticity. Cereb Cortex 25:1806–1814
Etiévant A, Manta S, Latapy C et al (2015) Repetitive transcranial magnetic stimulation induces long-lasting changes in protein expression and histone acetylation. Sci Rep 5:16873
Diaz Heijtz R, Almeida R, Eliasson AC et al (2018) Genetic variation in the dopamine system influences intervention outcome in children with cerebral palsy. EBioMedicine 28:162–167
Westin GG, Bassi BD, Lisanby SH et al (2014) Determination of motor threshold using visual observation overestimates transcranial magnetic stimulation dosage: safety implications. Clin Neurophysiol 125:142–147
Mir-Moghtadaei A, Caballero R, Fried P et al (2015) Concordance between BeamF3 and MRI-neuronavigated target sites for repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex. Brain Stimul 8:965–973
Fitzgerald PB, Hoy K, McQueen S et al (2009) A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology 34:1255–1262
Padala PR, Padala KP, Lensing SY et al (2018) Repetitive transcranial magnetic stimulation for apathy in mild cognitive impairment: a double-blind, randomized, sham-controlled, cross-over pilot study. Psychiatry Res 261:312–318
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF-2020R1A6A1A03043528 and NRF-2020M3E5D9080555 to IKL; 2019R1A2C1089515 to SML) and by the Health Fellowship Foundation to HH. The funders had no role study design, data collection, data analysis, data interpretation, manuscript preparation, manuscript review, manuscript approval, or decision to submit the manuscript for publication. The manuscript has not been previously published in print or electronic format and is not under consideration for publication elsewhere.
Funding
This article was funded by National Research Foundation of Korea (2020R1A6A1A0304352, 2020M3E5D9080555, 2019R1A2C1089515).
Author information
Authors and Affiliations
Contributions
IKL, SY, SML and SL designed the study. HH, RYK, SY, and SL acquired the data. HH, RYK, SML and SL analyzed the data. All authors interpreted the data. HH, RYK, SML and SL wrote the article. All authors reviewed the article. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study protocol was approved by the Institutional Review Board of Ewha W. University Mokdong Hospital.
Consent for participate
All patients provided written informed consent, in accordance with the 1964 Declaration of Helsinki.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hong, H., Kim, R.Y., Song, Y. et al. Genetic profile for dopamine signaling predicts brain functional reactivity to repetitive transcranial magnetic stimulation. Eur Arch Psychiatry Clin Neurosci 273, 99–111 (2023). https://doi.org/10.1007/s00406-022-01436-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-022-01436-2