Abstract
Up-regulation of the complement component 4A (C4A) in the brain has been associated with excessive synaptic pruning and increased schizophrenia (SZ) susceptibility. Over-expression of C4A has been observed in SZ postmortem brain tissue, and the gene encoding for a protein inhibitor of C4A activity, CUB and Sushi multiple domains 1 (CSMD1) gene, has been implicated in SZ risk and cognitive ability. Herein, we examined C4A and CSMD1 mRNA expression in peripheral blood from antipsychotic-naive individuals with first-episode psychosis (FEP; n = 73) and mentally healthy volunteers (n = 48). Imputed C4 locus structural alleles and C4A serum protein levels were investigated. Associations with symptom severity and cognitive domains performance were explored. A significant decrease in CSMD1 expression levels was noted among FEP patients compared to healthy volunteers, further indicating a positive correlation between C4A and CSMD1 mRNA levels in healthy volunteers but not in FEP cases. In addition, C4 copy number variants previously associated with SZ risk correlated with higher C4A mRNA levels in FEP cases, which confirms the regulatory effect of C4 structural variants on gene expression. Evidence also emerged for markedly elevated C4A serum concentrations in FEP cases. Within the FEP patient group, higher C4A mRNA levels correlated with more severe general psychopathology symptoms and lower CSMD1 mRNA levels predicted worse working memory performance. Overall, these findings suggest C4A complement pathway perturbations in individuals with FEP and corroborate the involvement of CSMD1 in prefrontal-mediated cognitive functioning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A large number of investigations have reported biochemical alterations of the immune system in patients with schizophrenia (SZ), providing support of the immune/inflammatory hypothesis for SZ as a putative pathophysiological mechanism increasing the vulnerability to the illness [1,2,3]. Similarly, dysregulated immune/inflammatory responses and differential expression of immune-related genes have been observed in individuals with first-episode of psychosis (FEP) [4,5,6,7], indicating that immune aberrations may exist even at the early stages of psychosis, further corroborating the view that underlying immunological deficiencies could play a role in the development or progression of psychotic disorders [8, 9]. In line with the above notion, genetic evidence has also emerged highlighting the involvement of genes coding for immune system components in SZ pathology [10, 11]. The involvement of complement system alterations in SZ etiopathogenesis, likely through the increased activity of the classical complement pathway, has long been considered an indication of disturbed innate immunity in SZ which may negatively affect neurodevelopmental processes [12]. On another front, the exacerbation of immune/inflammatory reactions during the acute phase of psychotic illness could be viewed as a compensatory or protective physiological mechanism that may alleviate psychotic symptoms and cognitive impairment [13]. Environmental influences and psychosocial distress may also induce secondary physiological processes including an increase of inflammatory responses [14, 15].
From a genomic perspective, fine-mapping of associations derived from large-scale genome-wide association studies (GWAS) in SZ, suggested that the complement component C4 constitutes a potential immune mediator which increases SZ susceptibility [11]. Specifically, structurally distinct alleles at the C4 gene locus, which encodes the complement component C4, have been genetically linked to SZ risk and associated with elevated C4A isotype gene expression in postmortem brain tissue from SZ patients. Over-expression of C4/C4A mRNA transcripts has been observed in multiple brain regions of SZ patients compared to healthy individuals [16, 17]. It is noted that C4 protein product is a member of the classical complement pathway that is activated during the innate immune response and forms a proteolytic protein cascade that clears cellular debris, enhances inflammation, and it is involved in the engulfment or elimination of pathogens [18]. Moreover, evidence from animal studies indicates that higher C4A isotype expression is implicated in excessive synaptic pruning in the brain, likely contributing to behavioral disturbances and cognitive deficits [19]. In accordance with the aforementioned findings, higher genetically predicted C4 gene expression is associated with poor memory performance in both SZ patients and healthy individuals [20]. With respect to differences in psychotic symptoms, increased C4A mRNA levels in peripheral blood cells have been shown to correlate with greater severity of psychopathology in SZ patients, specifically delusional symptoms [21]. Additionally, up-regulation of C4A protein levels has been found in plasma as well as in cerebrospinal fluid from patients with SZ [22,23,24,25]. Preliminary findings also suggest that higher total C4 plasma levels may predict unfavorable treatment response among FEP patients followed-up for twelve months [26].
It is of interest that among the strongest associated loci with SZ in recent GWAS is the CUB and Sushi Multiple Domains 1 (CSMD1) genetic locus, which encodes a protein inhibitor of C4 activity in neural tissues [27,28,29]. Further, reduced CSMD1 mRNA expression has been observed in peripheral blood of SZ patients [30] and common genetic variation within CSMD1 has been associated with psychosis proneness in the general population [31], as well as memory functioning in SZ and healthy individuals [32, 33]. Prompted by earlier findings implicating C4A and CSMD1 peripheral aberrations in SZ, the aim of the present study was to examine C4A and CSMD1 mRNA expression levels, as well as serum C4A protein levels, in peripheral blood from well-characterized antipsychotic-naïve FEP cases and mentally healthy individuals. In addition, genetically predicted C4 expression was estimated and we evaluated whether gene expression correlates with symptom severity at the early course of the illness and cognitive performance.
Materials and methods
Participants
In the current study, we included 73 unrelated cases (mean age 25.0 ± 7.2 years; 69% males) with non-affective first-episode psychosis (FEP) recruited as part of the collaborative Athens FEP Research Study [34]. Clinical diagnoses were established based on the International Classification of Diseases 10th Revision (ICD-10) diagnostic criteria (WHO, 1992) [35]. Consensus diagnoses for all cases were obtained from trained psychiatrists on the basis of detailed clinical records and individuals fulfilling diagnostic criteria for Schizophrenia-spectrum disorders (ICD-10 codes: F20-F29) were examined. All cases at the time of admission were antipsychotic-naïve and blood collection sampling was performed for basic biochemical examination and subsequent genomic analysis. Symptom severity at admission was assessed using the Positive and Negative Syndrome Scale (PANSS) [36]. Of the 73 FEP cases included in the study, 58 (79.5%) were hospitalized. Detailed demographic and clinical information is presented in Table S1. A total of 48 unrelated healthy volunteers (mean age 26.5 ± 4.8 years; 60.4% males) with no history of psychiatric disorder donated blood samples for annual routine biochemical examination and served as the control group. Each volunteer underwent a brief medical interview from trained physicians to assess the presence of major mental illness and other neurological or immunological disorders. Written informed consent was obtained from every individual after a detailed description of the research objectives and the study protocol was approved by the ethics committee and the Institutional Review Board at Eginition University Hospital (Athens, Greece).
Cognitive assessment
General cognitive ability was estimated using the Greek version of the Wechsler Adult Intelligence Scale (WAIS-Fourth edition) [37, 38], a comprehensive neurocognitive test comprising ten basic subtests grouped into four indexes representing distinct cognitive domains: verbal comprehension, perceptual reasoning, working memory and processing speed. Subtest raw scores were converted into age-corrected scaled scores using available Greek norms to determine the individual index scores and the full-scale IQ score. The assessment of neurocognitive functioning was performed within 3–4 weeks following admission to the research protocol by trained clinical neuropsychologists at Eginition University Hospital. In the current study, we enrolled 59 FEP cases with available neuropsychological data for further analysis.
RNA extraction and gene expression analysis
Total RNA was extracted from peripheral blood mononuclear cells (PBMCs) using the NucleoSpin RNA Blood kit (Macherey–Nagel, Düren, Germany), according to the protocol provided by the manufacturer. The purity and integrity of total RNA were evaluated using UV measurements and denaturing agarose gel electrophoresis, respectively. Reverse transcription (RT) reactions were performed using the PrimeScript First-Strand cDNA Synthesis Kit (TAKARA Bio, Japan) at 37 °C for 30 min followed by 85 °C for 5 s. The mRNA expression levels of C4A and CSMD1 were measured using semi-quantitative real-time polymerase chain reaction (RT-qPCR) on an ABI Prism 7000 instrument (Applied Biosystems, Foster City, CA, USA). Every cDNA sample was mixed with specific sets of primers and the qPCR master mix (KAPA SYBR FAST Universal Kit, Sigma-Aldrich, Germany) for 2 min at 50 °C and 2 min at 95 °C, followed by 40 cycles consisting of 15 s at 95 °C and 60 s at 60 °C. Finally, a standard dissociation protocol was used to ensure that each amplicon was a single product. All reactions were held in triplicate to ensure reproducible results. To evaluate differences in gene expression between groups, the fold change was calculated for each gene applying the comparative Ct (2−∆∆Ct) method. Relative mRNA expression levels were estimated by calculating delta Ct (Cycle threshold) values using the Ct values of the GAPDH housekeeping gene for normalization. Under-expressed genes are shown as the negative inverse of fold change and over-expressed as the fold change. Primers sequences were as follows: C4A forward, 5′-GGCTCACAGCCTTTGTGTTG-3′; C4A reverse, 5′-CCCTGCATGCTCCTGTCTAA-3′; CSMD1 forward, 5’-GTCTGGGCTCGTGGATATGT-3’; CSMD1 reverse, 5’-CAGGTCTCGGAAGGACAGAG-3’ and GAPDH forward, 5′-CGAGATCCCTCCAAAATCAA-3′; GAPDH reverse, 5′-TTCACACCCATGACGAACAT-3′.
Genotyping and C4 structural allele imputation
Genome-wide genotyping of unrelated FEP cases was performed using the Infinium Omni2.5 BeadChip array (Illumina Inc., San Diego, USA) at the Johns Hopkins Center for Inherited Disease Research (CIDR). Standard quality control (QC) processing steps of genotype data were performed using PLINK v1.90 [39]. High-quality genotyped single-nucleotide polymorphisms (SNPs) with call rate > 95%, minor allele frequency > 1%, Hardy–Weinberg equilibrium deviation p > 10–6 were retained for further analyses. Samples with low genotype rates (< 95%) were also excluded. To infer C4 structural alleles, 12,052 SNPs spanning the MHC region were extracted and utilized for imputation of C4 copy number structural variants following the procedures previously described by Sekar et al. (2016), using the MHC haplotypes HapMap3 CEU reference panel as recommended by the aforementioned group (http://mccarrolllab.org/resources/resources-for-c4/). Four common C4 haplotype groups were derived (BS, AL-BS, AL-BL, AL-AL) based on the combination of the C4 structural elements (C4A, C4B, C4L, C4S) that each individual carries and genetically predicted C4A expression levels were estimated in accordance with prior studies [11, 40]. In our FEP sample, the C4A predicted expression values ranged between 0 and 1.61 (mean: 1.26; standard deviation: 0.22).
Quantification of C4A serum levels
Human complement C4A protein levels in serum samples were determined using an enzyme-linked immunosorbent (ELISA) assay kit (AssayGenie, Dublin, Ireland) following the manufacturer’s instructions. Peripheral blood samples were obtained from every participant at admission (between 8.00 and 11.00 am) and centrifuged within 30 min after blood draw to collect serum samples for routine biochemical analysis. Serum aliquots were kept frozen at − 70 °C until further analysis. ELISA measurements were performed in a subset of FEP cases (n = 62) with available serum samples and triplicates were tested for each sample to calculate C4A mean concentration values for downstream statistical analyses.
Statistical analyses
Demographic characteristics were compared between groups by applying either Pearson’s chi-square (categorical variables) or Mann–Whitney U tests (continuous variables), as appropriate. To evaluate gene expression differences between FEP cases and healthy volunteers, the comparative Ct method (2−∆∆Ct) was utilized and fold-change differences were calculated. Differences in relative mRNA expression levels (log-transformed ΔCt values) between groups and C4A serum levels were examined using one-sided Mann–Whitney U tests, as prior evidence has shown altered expression levels of C4A and CSMD1 in SZ [11, 16, 30]. Within-group correlations between C4A and CSMD1 relative gene expression were tested by applying Spearman’s rank correlation coefficients (rho). Linear regression models adjusted for age and gender were applied to test for associations between relative gene expression levels and PANSS subscale scores or WAIS-IV cognitive domains scores. Correlations between genetically predicted C4 copy number structural alleles (C4 haplotype groups) and mRNA transcript levels were estimated by linear regression analysis as previously reported (40). The significance threshold was set at p < 0.05. All analyses were performed using R version 4.1.2 package.
Results
Demographic and clinical characteristics
In the present study, we included a total of 73 FEP cases and 48 mentally healthy volunteers. Detailed demographic and clinical information for the FEP group of cases is presented in Supplementary Table S1. No significant differences were observed with regard to gender (χ2 = 0.515; p = 0.473) or age (Mann–Whitney test p = 0.205) between FEP and healthy groups. All FEP cases were antipsychotic-naïve at the time of inclusion to the study and 80% were hospitalized. According to the ICD-10 diagnostic criteria, 88% of cases received a Schizophrenia (SZ) diagnosis (F20 ICD-10 code). The remaining cases (12%) were classified as psychosis-spectrum disorders (F23 or F28 ICD-10 codes; Supplementary Table S1).
C4A and CSMD1 mRNA expression levels in peripheral blood
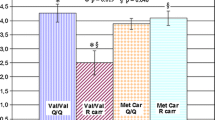
The relative expression of C4A and CSMD1 mRNA levels in PBMCs was compared between FEP cases and healthy volunteers. As shown in Fig. 1, we observed that C4A was marginally over-expressed in FEP cases; however, this difference did not reach statistical significance (one-sided Mann–Whitney test p = 0.132). CSMD1 gene expression was found significantly reduced among FEP cases compared to healthy volunteers (1.4-fold change; one-sided Mann–Whitney test p = 0.004). Female FEP cases showed a trend toward higher C4A expression levels compared to male cases (Mann–Whitney test p = 0.067), whereas the above association was not present in healthy participants (Mann–Whitney test p = 0.749). Sex differences were not observed for CSMD1 expression levels in both FEP and healthy groups. Furthermore, all the above associations remained unchanged after adjustment for ICD-10-based diagnostic classifications. Within-group analyses revealed a significant positive correlation between C4A and CSMD1 mRNA expression levels in healthy volunteers (rho = 0.40, p = 0.005), yet this relationship was not detected in FEP cases (rho = 0.16, p = 0.197).
C4 structural genetic variation correlates with C4A mRNA levels
We further examined the correlation between C4A/C4B locus structural alleles (haplotype groups: BS, AL-BS, AL-BL, AL-AL) and measured mRNA levels in peripheral PBMCs, in an effort to validate previous findings in the human brain [11], demonstrating an association between genetically predicted C4A/C4B haplotype status and gene expression. Our results indicated that among FEP cases (n = 71), carriers of the SZ risk AL-AL haplotype showed higher C4A mRNA levels (β = 0.26; p = 0.02) (Fig. 2), whereas no association was found with CSMD1 mRNA levels (β = − 0.03; p = 0.817), as expected. In agreement with recent evidence from an independent FEP study [22], we observed that the AL-BL haplotype group was the most common among FEP cases (56%), compared to a much lower frequency reported within healthy individuals (41%) in the original study by Sekar et al. (2016). Likewise, the SZ low risk BS haplogroup (7% frequency in the Sekar et al. study) reached a comparable frequency in this FEP sample (8.2%).
Elevated C4A serum levels in FEP cases
The C4A protein concentrations were found significantly elevated in FEP cases compared to healthy volunteers (mean FEP: 999.5 ± 160.9 ng/ml; mean healthy: 519.4 ± 172.6 ng/ml, Mann–Whitney test p < 10–6; Fig. 3). C4A serum levels did not differ significantly by gender (χ2 = 115.8; p = 0.359) or age (Mann–Whitney test p = 0.906). We also noted that C4A blood mRNA levels did not correlate significantly with C4A serum levels in our sample (β = 0.12; p = 0.421). Similarly, genetically predicted C4A expression (C4 copy number variation) was not associated with C4A protein levels (β = 0.05; p = 0.747).
Associations with symptom severity and cognitive performance
Within-group analyses were applied to evaluate the potential impact of C4A and CSMD1 gene expression on PANSS subscales scores at admission as well as cognitive performance in FEP cases. As shown in Fig. 4, significant positive correlations were observed between C4A mRNA expression levels and PANSS general psychopathology (β = 0.29; p = 0.016) and total (β = 0.28; p = 0.019) symptom scores. With regard to cognitive performance, increased CSMD1 mRNA levels were associated with better performance on working memory (β = 0.27; p = 0.037). C4A mRNA and C4A serum protein levels did not correlate significantly with either PANSS baseline subscales scores or cognitive indices (Fig. 5; Supplementary Table S2).
Discussion
The results of the present study add to a growing body of evidence implicating aberrant immune/inflammatory responses in patients with SZ and those experiencing FEP [1, 4,5,6, 26, 41]. We provide evidence for common transcriptional regulation of C4A and CSMD1 among healthy individuals, suggesting co-expression of the two genes and related biological functions in the C4A-dependent complement pathway. Importantly, our results indicate C4A cascade alterations in un-medicated cases with FEP, suggesting an abnormal innate immune reaction during the early course of psychosis [5, 26]. It is stressed, though, that there is a difficulty to precisely identify the exact etio-pathological mechanisms which induce C4 complement activation and related immunological dysregulation in FEP. The relationship between environmental stress or adverse life events and the enhancement of immune/inflammatory responses has been documented in psychotic disorders [14, 42, 43], and plausibly explains the immune exacerbations following an acute episode of psychosis [13]. Moreover, up-regulation of the complement system has been observed in rodents following exposure to stressful conditions [44]. The observed gene expression differences in FEP patients are in accordance with previous studies indicating higher C4/C4A expression levels in postmortem brain tissue [11, 16, 17] and significantly lower CSMD1 expression levels in peripheral blood from SZ patients [30]. A trend for increased C4A expression levels in PBMCs, although not statistically significant, has been observed in an earlier study which examined C4A blood mRNA levels in patients with SZ and psychotic bipolar disorder under medication with antipsychotics [21]. We argue that antipsychotic treatment could potentially impact gene expression levels; therefore, future investigations with larger sample sizes and well-characterized un-medicated patients are needed to delineate whether increased C4A expression stems from illness specific etio-pathological mechanisms or unidentified non-specific drug-induced phenomena.
This is to the best of our knowledge the first study reporting C4A isotype mRNA expression levels in peripheral blood in relation to genetically predicted C4A gene expression. In particular, we validate the positive correlation previously seen in brain postmortem tissue between SZ risk increasing C4A structural variation (i.e., AL-AL haplotype) and experimentally determined mRNA levels in immune cells [11]. The above observation supports the regulatory effect of distinct C4A copy number variants on gene transcription and has significant implications for future genetic studies aiming to estimate C4A expression profiles and investigate the contribution of deregulated C4/C4A expression on psychotic disorders. It is of interest that prior evidence has shown that genetically predicted C4A expression associates with cognitive impairment in SZ patients and differences in brain imaging measures, such as cortical activation and thickness in healthy individuals [20, 40]. In this work, there was no indication for a correlation between either predicted C4 structural alleles or measured mRNA levels and cognitive performance among FEP cases. Notably, the most frequent C4 structural variant, that is AL-BL haplogroup, was associated with higher SZ risk in the original study by Sekar et al. (2016) and was found to predispose to microbial infections in SZ patients [45], which naturally could activate innate immune responses and complement system up-regulation.
In addition, we provide evidence that FEP cases are characterized by highly elevated serum C4A protein levels, compared to mentally healthy volunteers. This observation confirms the results of an earlier study reporting increased C4A levels in SZ patients measured by highly sensitive mass spectrometry methodology [23]. It is mentioned, however, that most studies so far have estimated peripheral total C4 protein levels in cases diagnosed with FEP or SZ reporting no significant differences in serum [24, 25, 46], but elevated levels in cerebrospinal fluid [24]. Our findings outline that a specific dysregulation of C4A isotype levels may exist in psychosis, at least in the very early stages of the illness, which could not be detected using methods that estimate total amount of complement C4 (i.e., C4A and C4B levels) [6]. It remains to be determined in future studies whether the observed increase in C4A protein levels is attributed to underlying biochemical alterations with considerable biological impact on the disposition to psychosis. To this perspective, findings from FEP cases followed-up for an entire year showed that higher baseline C4 serum levels may represent an early indicator of poor treatment response [26], suggesting that peripheral C4 concentration could potentially inform clinicians for optimal treatment intervention in individuals with FEP. Our analyses did not reveal significant correlation between C4A serum protein and mRNA levels in this population, which is possibly attributed to complicated cellular mechanisms involved in gene transcription regulation, mRNA processing and protein synthesis rates [47, 48]. Moreover, we acknowledge as an additional limiting factor the slightly smaller number of FEP cases included in the C4A protein assays that might have reduced the statistical power of the analysis. Likewise, C4 haplogroup analysis among FEP cases did not reveal an association between C4A copy number status and C4A protein levels in serum, as opposed to an earlier study which examined a limited number of plasma samples from medicated SZ patients [22].
Prompted by previous reports supporting a relationship between C4A and CSMD1 gene expression deviations and symptom dimensions as well as cognitive function [20, 21, 30, 49], we attempted to assess the relationships with symptom severity and cognitive performance in cases with FEP. In our sample, nominally significant associations were noted between C4A peripheral mRNA levels and PANSS general psychopathology as well as total symptoms severity, which corroborate prior evidence indicating a relationship between higher C4A expression levels in PBMCs and more severe psychotic symptomatology [21], albeit it should be stressed that our limited sample size could not permit definite conclusions. Further, recent findings from a FEP cohort did not observe correlations between genetically predicted C4 expression by estimating C4 haplotype status and symptom severity [22], implying that additional evidence is essential to elucidate whether altered C4/C4A expression could prove as a meaningful clinical biomarker [50].
Importantly, our findings indicate that lower peripheral CSMD1 expression levels correlate with poor performance on prefrontal-mediated cognitive domains, in particular perceptual reasoning. Common genetic variation within CSMD1 has been credibly associated with SZ in recent GWAS meta-analyses [51], and implicated in human cognitive impairment as well as reduced brain functional activation [33, 52]. We postulate that lower CSMD1 expression negatively impacts on cognitive functioning in FEP cases by compromising C4-dependent complement pathway, which has been involved in synaptic pruning processes and neurodevelopmental mechanisms [11, 53]. It is noteworthy to mention that functional studies have reported that CSMD1 constitutes a complement cascade regulatory factor, acting as an inhibitor of C4-dependent cellular processes in neural tissues [28, 29]. The biological relationship between C4A and CSMD1 components and their role in shared biochemical processes of the complement system is strengthened by studies examining psychosis-related behavioral and cognitive phenotypes in mice. Specifically, over-expression of C4/C4A components might contribute to the enhancement of anxiety-like behaviors, social impairment and working memory deficits [19, 54], whereas depletion of the CSMD1 genetic locus induces emotional and cognitive defects [55]. Therefore, it becomes evident that C4A and CSMD1 complement factors likely operate in common cellular pathways with opposing functions [29]. To this perspective, the observed inverse direction of gene expression patterns for C4A and CSMD1 in FEP cases denotes a transcriptional dysregulation of the two complement-related genes at psychosis onset and/or at a drug-naïve state.
In conclusion, this study suggests a hyperactivity of the C4/C4A complement pathway in un-medicated individuals diagnosed with FEP, predominantly SZ [12, 56]. Furthermore, the results add further support to an altered immune/inflammatory response at least in a subset of individuals with FEP, which might contribute to the development of psychotic symptoms [5, 6, 9]. The exact biological importance of complement pathway dysregulation in the occurrence of psychosis is still not well understood [50], although recent evidence has implicated complement system alterations to early-life brain synaptic abnormalities and neurodevelopmental deficits, perhaps related to increased SZ predisposition [53, 56, 57]. Additional research efforts aiming to characterize the immune/inflammatory profile early in the course of psychotic illness may shed more light on the influence of complement factors in the pathogenesis of SZ and related psychosis-spectrum disorders [56].
References
Muller N (2018) Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr Bull 44:973–982
van Kesteren CF, Gremmels H, de Witte LD, Hol EM, Van Gool AR, Falkai PG, Kahn RS, Sommer IE (2017) Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry 7:e1075
Buckley PF (2019) Neuroinflammation and schizophrenia. Curr Psychiatry Rep 21:72
Pillinger T, Osimo EF, Brugger S, Mondelli V, McCutcheon RA, Howes OD (2019) A meta-analysis of immune parameters, variability, and assessment of modal distribution in psychosis and test of the immune subgroup hypothesis. Schizophr Bull 45:1120–1133
Kopczynska M, Zelek W, Touchard S, Gaughran F, Di Forti M, Mondelli V, Murray R, O’Donovan MC, Morgan BP (2019) Complement system biomarkers in first episode psychosis. Schizophr Res 204:16–22
Steiner J, Frodl T, Schiltz K, Dobrowolny H, Jacobs R, Fernandes BS, Guest PC, Meyer-Lotz G, Borucki K, Bahn S, Bogerts B, Falkai P, Bernstein HG (2020) Innate immune cells and C-reactive protein in acute first-episode psychosis and schizophrenia: relationship to psychopathology and treatment. Schizophr Bull 46:363–373
Enrico P, Delvecchio G, Turtulici N, Pigoni A, Villa FM, Perlini C, Rossetti MG, Bellani M, Lasalvia A, Bonetto C, Scocco P, D’Agostino A, Torresani S, Imbesi M, Bellini F, Veronese A, Bocchio-Chiavetto L, Gennarelli M, Balestrieri M, Colombo GI, Brambilla P (2021) Classification of psychoses based on immunological features: a machine learning study in a large cohort of first-episode and chronic patients. Schizophr Bull 47:1141–1151
Mongan D, Focking M, Healy C, Susai SR, Heurich M, Wynne K, Nelson B, McGorry PD, Amminger GP, Nordentoft M, Krebs MO, Riecher-Rossler A, Bressan RA, Barrantes-Vidal N, Borgwardt S, Ruhrmann S, Sachs G, Pantelis C, van der Gaag M, de Haan L, European Network of National Schizophrenia Networks Studying Gene-Environment Interactions (EU-GEI) High Risk Study Group (2021) Development of proteomic prediction models for transition to psychotic disorder in the clinical high-risk state and psychotic experiences in adolescence. JAMA Psychiatry 78:77–90
Murray RM, Mondelli V, Stilo SA, Trotta A, Sideli L, Ajnakina O, Ferraro L, Vassos E, Iyegbe C, Schoeler T, Bhattacharyya S, Marques TR, Dazzan P, Lopez-Morinigo J, Colizzi M, O’Connor J, Falcone MA, Quattrone D, Rodriguez V, Tripoli G, Di Forti M (2020) The influence of risk factors on the onset and outcome of psychosis: what we learned from the GAP study. Schizophr Res 225:63–68
Pouget JG (2018) The emerging immunogenetic architecture of schizophrenia. Schizophr Bull 44:993–1004
Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, Genovese G, Rose SA, Handsaker RE, Daly MJ, Carroll MC, Stevens B, McCarroll SA, Schizophrenia Working Group of the Psychiatric Genomics Consortium (2016) Schizophrenia risk from complex variation of complement component 4. Nature 530:177–183
Mayilyan KR, Weinberger DR, Sim RB (2008) The complement system in schizophrenia. Drug News Perspect 21:200–210
Roomruangwong C, Noto C, Kanchanatawan B, Anderson G, Kubera M, Carvalho AF, Maes M (2020) The role of aberrations in the immune-inflammatory response system (IRS) and the compensatory immune-regulatory reflex system (CIRS) in different phenotypes of schizophrenia: the IRS-CIRS theory of schizophrenia. Mol Neurobiol 57:778–797
Howes OD, McCutcheon R (2017) Inflammation and the neural diathesis-stress hypothesis of schizophrenia: a reconceptualization. Transl Psychiatry 7:e1024
Nettis MA, Pariante CM, Mondelli V (2020) Early-life adversity, systemic inflammation and comorbid physical and psychiatric illnesses of adult life. Curr Top Behav Neurosci 44:207–225
Rey R, Suaud-Chagny MF, Bohec AL, Dorey JM, d’Amato T, Tamouza R, Leboyer M (2020) Overexpression of complement component C4 in the dorsolateral prefrontal cortex, parietal cortex, superior temporal gyrus and associative striatum of patients with schizophrenia. Brain Behav Immun 90:216–225
Purves-Tyson TD, Robinson K, Brown AM, Boerrigter D, Cai HQ, Weissleder C, Owens SJ, Rothmond DA, Shannon Weickert C (2020) Increased macrophages and C1qA, C3, C4 transcripts in the midbrain of people with schizophrenia. Front Immunol 11:2002
Nesargikar PN, Spiller B, Chavez R (2012) The complement system: history, pathways, cascade and inhibitors. Eur J Microbiol Immunol 2:103–111
Yilmaz M, Yalcin E, Presumey J, Aw E, Ma M, Whelan CW, Stevens B, McCarroll SA, Carroll MC (2021) Overexpression of schizophrenia susceptibility factor human complement C4A promotes excessive synaptic loss and behavioral changes in mice. Nat Neurosci 24:214–224
Donohoe G, Holland J, Mothersill D, McCarthy-Jones S, Cosgrove D, Harold D, Richards A, Mantripragada K, Owen MJ, O’Donovan MC, Gill M, Corvin A, Morris DW, WTCCC2 (2018) Genetically predicted complement component 4A expression: effects on memory function and middle temporal lobe activation. Psychol Med 48:1608–1615
Melbourne JK, Rosen C, Feiner B, Sharma RP (2018) C4A mRNA expression in PBMCs predicts the presence and severity of delusions in schizophrenia and bipolar disorder with psychosis. Schizophr Res 197:321–327
Kalinowski A, Liliental J, Anker LA, Linkovski O, Culbertson C, Hall JN, Pattni R, Sabatti C, Noordsy D, Hallmayer JF, Mellins ED, Ballon JS, O’Hara R, Levinson DF, Urban AE (2021) Increased activation product of complement 4 protein in plasma of individuals with schizophrenia. Transl Psychiatry 11(1):486
Walss-Bass C, Lokesh G, Dyukova E, Gorenstein DG, Roberts DL, Velligan D, Volk DE (2019) X-aptamer technology identifies C4A and ApoB in blood as potential markers for schizophrenia. Mol Neuropsychiatry 5:52–59
Gallego JA, Blanco EA, Morell C, Lencz T, Malhotra AK (2021) Complement component C4 levels in the cerebrospinal fluid and plasma of patients with schizophrenia. Neuropsychopharmacology 46:1140–1144
Laskaris L, Zalesky A, Weickert CS, Di Biase MA, Chana G, Baune BT, Bousman C, Nelson B, McGorry P, Everall I, Pantelis C, Cropley V (2019) Investigation of peripheral complement factors across stages of psychosis. Schizophr Res 204:30–37
Mondelli V, Di Forti M, Morgan BP, Murray RM, Pariante CM, Dazzan P (2020) Baseline high levels of complement component 4 predict worse clinical outcome at 1-year follow-up in first-episode psychosis. Brain Behav Immun 88:913–915
Escudero-Esparza A, Kalchishkova N, Kurbasic E, Jiang WG, Blom AM (2013) The novel complement inhibitor human CUB and Sushi multiple domains 1 (CSMD1) protein promotes factor I-mediated degradation of C4b and C3b and inhibits the membrane attack complex assembly. FASEB 27:5083–5093
Kraus DM, Elliott GS, Chute H, Horan T, Pfenninger KH, Sanford SD, Foster S, Scully S, Welcher AA, Holers VM (2006) CSMD1 is a novel multiple domain complement-regulatory protein highly expressed in the central nervous system and epithelial tissues. J Immunol 176:4419–4430
Baum ML, Wilton DK, Muthukumar A, Fox RG, Carey A, Crotty W, Scott-Hewitt N, Bien E, Sabatini DA, Lanser T, Frouin A, Gergits F, Havik B, Gialeli C, Nacu E, Blom AM, Eggan K, Johnson MB, McCarroll SA, Stevens B (2020) CUB and Sushi multiple domains 1 (CSMD1) opposes the complement cascade in neural tissues. bioRxiv. https://doi.org/10.1101/2020.09.11.291427
Liu Y, Fu X, Tang Z, Li C, Xu Y, Zhang F, Zhou D, Zhu C (2019) Altered expression of the CSMD1 gene in the peripheral blood of schizophrenia patients. BMC Psychiatry 19:113
Ortega-Alonso A, Ekelund J, Sarin AP, Miettunen J, Veijola J, Jarvelin MR, Hennah W (2017) Genome-wide association study of psychosis proneness in the Finnish population. Schizophr Bull 43:1304–1314
Donohoe G, Walters J, Hargreaves A, Rose EJ, Morris DW, Fahey C, Bellini S, Cummins E, Giegling I, Hartmann AM, Moller HJ, Muglia P, Owen MJ, Gill M, O’Donovan MC, Tropea D, Rujescu D, Corvin A (2013) Neuropsychological effects of the CSMD1 genome-wide associated schizophrenia risk variant rs10503253. Genes Brain Behav 12:203–209
Athanasiu L, Giddaluru S, Fernandes C, Christoforou A, Reinvang I, Lundervold AJ, Nilsson LG, Kauppi K, Adolfsson R, Eriksson E, Sundet K, Djurovic S, Espeseth T, Nyberg L, Steen VM, Andreassen OA, Le Hellard S (2017) A genetic association study of CSMD1 and CSMD2 with cognitive function. Brain Behav Immun 61:209–216
Xenaki LA, Kollias CT, Stefanatou P, Ralli I, Soldatos RF, Dimitrakopoulos S, Hatzimanolis A, Triantafyllou TF, Kosteletos I, Vlachos II, Selakovic M, Foteli S, Mantonakis L, Ermiliou V, Voulgaraki M, Psarra E, Guloksuz S, van Os J, Stefanis NC (2020) Organization framework and preliminary findings from the Athens first-episode psychosis research study. Early Interv Psychiatry 14:343–355
World Health Organization (1992) The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines, vol 1992. World Health Organization, Geneva
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13(2):261–276
Stogiannidou A (2011) WAIS-IV GR (Wechsler adult intelligence scale), 4th edn. Motivo Publications, Athens
Wechsler D (2008) Wechsler adult intelligence scale, 4th edn. Pearson, San Antonio
Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ (2015) Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience 4:7
O’Connell KS, Sonderby IE, Frei O, van der Meer D, Athanasiu L, Smeland OB, Alnaes D, Kaufmann T, Westlye LT, Steen VM, Andreassen OA, Hughes T, Djurovic S (2021) Association between complement component 4A expression, cognitive performance and brain imaging measures in UK Biobank. Psychol Med. https://doi.org/10.1017/S0033291721000179
Mariaselvam CM, Wu CL, Boukouaci W, Richard JR, Barau C, Le Corvoisier F, Dazzan P, Egerton A, Pollak TA, McGuire P, Rujescu D, Jamain S, Leboyer M, Tamouza R (2021) The complement C4 genetic diversity in first episode psychosis of the OPTiMiSE cohort. Schizophr Bull Open 2:sga003
van Mierlo HC, Schot A, Boks M, de Witte LD (2020) The association between schizophrenia and the immune system: review of the evidence from unbiased “omic-studies.” Schizophr Res 217:114–123
Radhakrishnan R, Kaser M, Guloksuz S (2017) The link between the immune system, environment, and psychosis. Schizophr Bull 43:693–697
Focking M, Sabherwal S, Cates HM, Scaife C, Dicker P, Hryniewiecka M, Wynne K, Rutten B, Lewis G, Cannon M, Nestler EJ, Heurich M, Cagney G, Zammit S, Cotter DR (2021) Complement pathway changes at age 12 are associated with psychotic experiences at age 18 in a longitudinal population-based study: evidence for a role of stress. Mol Psychiatry 26:524–533
Severance EG, Leister F, Lea A, Yang S, Dickerson F, Yolken RH (2021) Complement C4 associations with altered microbial biomarkers exemplify gene-by-environment interactions in schizophrenia. Schizophr Res 234:87–9348
Mongan D, Sabherwal S, Susai SR, Focking M, Cannon M, Cotter DR (2020) Peripheral complement proteins in schizophrenia: a systematic review and meta-analysis of serological studies. Schizophr Res 222:58–72
Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M (2011) Global quantification of mammalian gene expression control. Nature 473:337–342
Liu Y, Beyer A, Aebersold R (2016) On the dependency of cellular protein levels on mRNA abundance. Cell 165:535–550
Koiliari E, Roussos P, Pasparakis E, Lencz T, Malhotra A, Siever LJ, Giakoumaki SG, Bitsios P (2014) The CSMD1 genome-wide associated schizophrenia risk variant rs10503253 affects general cognitive ability and executive function in healthy males. Schizophr Res 154:42–47
Lizano P, Prasad KM, Keshavan MS (2019) Commentary: do complement factors “connect the dots” in schizophrenia? Schizophr Res 204:4–6
Pardinas AF, Holmans P, Pocklington AJ, Escott-Price V, Ripke S, Carrera N, Legge SE, Bishop S, Cameron D, Hamshere ML, Han J, Hubbard L, Lynham A, Mantripragada K, Rees E, MacCabe JH, McCarroll SA, Baune BT, Breen G, Byrne EM, Walters J (2018) Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet 50:381–389
Rose EJ, Morris DW, Hargreaves A, Fahey C, Greene C, Garavan H, Gill M, Corvin A, Donohoe G (2013) Neural effects of the CSMD1 genome-wide associated schizophrenia risk variant rs10503253. Am J Med Genet 162B:530–537
Keshavan MS, Anderson S, Pettegrew JW (1994) Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res 28:239–265
Druart M, Nosten Bertrand M, Poll S, Crux S, Nebeling F, Delhaye C, Dubois Y, Leboyer M, Tamouza R, Fuhrmann M, LeMagueresse C (2021) Elevated expression of complement C4 in the mouse prefrontal cortex causes schizophrenia-associated phenotypes. Mol Psychiatry 26:3489–3501
Steen VM, Nepal C, Ersland KM, Holdhus R, Naevdal M, Ratvik SM, Skrede S, Havik B (2013) Neuropsychological deficits in mice depleted of the schizophrenia susceptibility gene CSMD1. PloS One 8:e79501
Woo JJ, Pouget JG, Zai CC, Kennedy JL (2020) The complement system in schizophrenia: where are we now and what’s next? Mol Psychiatry 25:114–130
Sager R, Walker AK, Middleton F, Robinson K, Webster MJ, Weickert CS (2020) Trajectory of change in brain complement factors from neonatal to young adult humans. J Neurochem. https://doi.org/10.1111/jnc.15241
Acknowledgements
The authors are thankful to the participants of the present study and their family members for the valuable information they kindly provided.
Funding
This study was supported by research funding from the Theodor-Theohari Cozzika Foundation (Athens, Greece).
Author information
Authors and Affiliations
Contributions
AH, MG and NCS conceived and designed the study. SF, CN and KK participated in involving the subjects. SF, PS, AEN, KK and NCS participated in clinical and neuropsychological assessments and data acquisition and management. AH and MG participated in data analysis. AH and MG wrote the first draft of the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no biomedical financial interests or potential conflicts of interest.
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Clinical Research Ethics committee of Eginition University Hospital, National and Kapodistrian University of Athens.
Consent to participate
All participants signed an informed consent prior to their inclusion to the study.
Consent for publication
All participants signed an informed consent regarding publishing their data.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hatzimanolis, A., Foteli, S., Stefanatou, P. et al. Deregulation of complement components C4A and CSMD1 peripheral expression in first-episode psychosis and links to cognitive ability. Eur Arch Psychiatry Clin Neurosci 272, 1219–1228 (2022). https://doi.org/10.1007/s00406-022-01409-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-022-01409-5