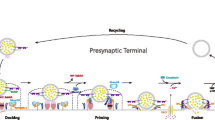
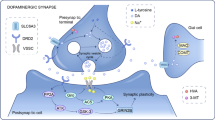
Abstract
It was presumed syntaxin-1A (STX1A) might relate to the pathophysiology of attention-deficit/hyperactivity disorder (ADHD), but the results were inconsistent. The present study aims to confirm whether the STX1A gene is involved in the susceptibility of children ADHD. We genotyped three single nucleotide polymorphisms (SNPs) of STX1A gene using Sequenom MassARRAY technology. A case–control study was performed among Chinese Han population including 754 cases and 772 controls from two different provinces. The Conners Parent Symptom Questionnaire and Integrated Visual and Auditory Continuous Performance Test were used to assess ADHD clinical symptoms. We found for the first time that rs3793243 GG genotype carriers had a lower risk of ADHD compared with AA genotype (OR 0.564, 95% confidence interval (CI) 0.406–0.692, P = 0.001), and rs875342 was also associated with children ADHD (OR 1.806, 95% CI 1.349–2.591, P = 0.001). In addition, the two positive SNPs were also significantly associated with the clinical characteristics of ADHD. Expression quantitative trait loci analysis indicated that rs3793243 might mediate STX1A gene expression. Using a case–control study to explore the association between STX1A gene and children ADHD in Chinese Han population, our results suggest STX1A genetic variants might contribute to the susceptibility of children ADHD.



Similar content being viewed by others
References
Austerman J (2015) ADHD and behavioral disorders: Assessment, management, and an update from DSM-5. Clevel Clin J Med 82(11 Suppl 1):S2–7
Willcutt EG (2012) The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics 9(3):490–499
GBD 2015 Disease and Injury Incidence and Prevalence Collaborators (2015) Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388(10053):1545–1602
Singh I (2008) Beyond polemics: science and ethics of ADHD. Nat Rev Neurosci 9(12):957–964
Biederman J, Faraone SV (2005) Attention-deficit hyperactivity disorder. Lancet 366(9481):237–248
Kooij SJ et al (2010) European consensus statement on diagnosis and treatment of adult ADHD: The European Network Adult ADHD. BMC Psychiatry 10:67
Millichap JG (2011) Attention deficit hyperactivity disorder handbook. Springer, New York, pp 791–792
Thapar A et al (2013) What have we learnt about the causes of ADHD? J Child Psychol Psychiatry 54(1):3–16
Faraone SV, Mick E (2010) Molecular genetics of attention deficit hyperactivity disorder. Psychiatr Clin N Am 33(1):159–180
Bokor G, Anderson PD (2014) Attention-deficit/hyperactivity disorder. J Pharm Pract 27(4):336–349
Elia J et al (2010) Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Mol Psychiatry 15(6):637–646
Lin RC, Scheller RH (2000) Mechanisms of synaptic vesicle exocytosis. Annu Rev Cell Dev Biol 16:19–49
Bennett MK, Calakos N, Scheller RH (1992) Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science 257(5067):255–259
Oyler GA et al (1989) The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol 109(6 Pt 1):3039–3052
Trimble WS, Cowan DM, Scheller RH (1988) VAMP-1: a synaptic vesicle-associated integral membrane protein. Proc Natl Acad Sci USA 85(12):4538–4542
Zylbersztejn K, Galli T (2011) Vesicular traffic in cell navigation. FEBS J 278(23):4497–4505
Feng Y et al (2005) The SNAP25 gene as a susceptibility gene contributing to attention-deficit hyperactivity disorder. Mol Psychiatry 10(11):998–1005
Mill J et al (2005) Quantitative trait locus analysis of candidate gene alleles associated with attention deficit hyperactivity disorder (ADHD) in five genes: DRD4, DAT1, DRD5, SNAP-25, and 5HT1B. Am J Med Genet B Neuropsychiatr Genet 133b(1):68–73
Kim JW et al (2007) Investigation of variation in SNAP-25 and ADHD and relationship to co-morbid major depressive disorder. Am J Med Genet B Neuropsychiatr Genet 144b(6):781–790
Guan L et al (2009) A high-density single-nucleotide polymorphism screen of 23 candidate genes in attention deficit hyperactivity disorder: suggesting multiple susceptibility genes among Chinese Han population. Mol Psychiatry 14(5):546–554
Forero DA et al (2009) Candidate genes involved in neural plasticity and the risk for attention-deficit hyperactivity disorder: a meta-analysis of 8 common variants. J Psychiatry Neurosci 34(5):361–366
Zhang H et al (2011) An association study between SNAP-25 gene and attention-deficit hyperactivity disorder. Eur J Paediatr Neurol 15(1):48–52
Sarkar K et al (2012) Role of SNAP25 explored in eastern Indian attention deficit hyperactivity disorder probands. Neurochem Res 37(2):349–357
Hawi Z et al (2013) DNA variation in the SNAP25 gene confers risk to ADHD and is associated with reduced expression in prefrontal cortex. PLoS ONE 8(4):e60274
Neale BM et al (2008) Genome-wide association scan of attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 147B(8):1337–1344
Lasky-Su J et al (2008) Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. Am J Med Genet B Neuropsychiatr Genet 147b(8):1345–1354
Neale BM et al (2010) Case–control genome-wide association study of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 49(9):906–920
Yang L et al (2013) Polygenic transmission and complex neuro developmental network for attention deficit hyperactivity disorder: genome-wide association study of both common and rare variants. Am J Med Genet B Neuropsychiatr Genet 162B(5):419–430
Mooney MA et al (2016) Pathway analysis in attention deficit hyperactivity disorder: an ensemble approach. Am J Med Genet Part B Neuropsychiatr Genet 171(6):815–826
Gao Q et al (2015) Synaptosome-related (SNARE) genes and their interactions contribute to the susceptibility and working memory of attention-deficit/hyperactivity disorder in males. Prog Neuropsychopharmacol Biol Psychiatry 57:132–139
Brookes KJ et al (2005) DNA pooling analysis of ADHD and genes regulating vesicle release of neurotransmitters. Am J Med Genet B Neuropsychiatr Genet 139b(1):33–37
Brookes K et al (2006) The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry 11(10):934–953
Sanchez-Mora C et al (2013) Evaluation of common variants in 16 genes involved in the regulation of neurotransmitter release in ADHD. Eur Neuropsychopharmacol 23(6):426–435
Gong YX, Cai TS (1994) Chinese wechsler intelligence scale for children—revised, vol 2. Map Press Hunan, China, pp 1–6
Springer, U.S. (2011) Conners’ parent rating scale: revised, vol 404. Springer US, New York
Fan J (2005) The norm and reliability of the conners parent symptom questionnaire in Chinese urban children. Shanghai Arch Psychiatry 17(6):321–323
Morgan JF (2007) p Value fetishism and use of the Bonferroni adjustment. Evid Based Mental Health 10(2):34–35
Lubin JH, Gail MH (1990) On power and sample size for studying features of the relative odds of disease. Am J Epidemiol 131(3):552–566
Kenar AN et al (2014) Association of VAMP-2 and Syntaxin 1A genes with adult attention deficit hyperactivity disorder. Psychiatry Investig 11(1):76–83
Olgiati P et al (2014) Role of synaptosome-related (SNARE) genes in adults with attention deficit hyperactivity disorder. Psychiatry Res 215(3):799–800
Neale BM et al (2010) Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 49(9):884–897
Demontis D et al (2019) Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet 51(1):63–75
Igarashi M et al (1996) 1224 A t-Snare is involved in axonal growth: botulinum neurotoxin C1 induces growth cone collapse. Neurosci Res 25(Suppl1):S134
Yamaguchi K et al (1996) Enhancement of neurite-sprouting by suppression of HPC-1/syntaxin 1A activity in cultured vertebrate nerve cells. Brain Res 740(1–2):185–192
Fernandez I et al (1998) Three-dimensional structure of an evolutionarily conserved N-terminal domain of syntaxin 1A. Cell 94(6):841–849
Shimojo M et al (2015) SNAREs controlling vesicular release of BDNF and development of callosal axons. Cell Rep 11(7):1054–1066
Fujiwara T et al (2006) Analysis of knock-out mice to determine the role of HPC-1/syntaxin 1A in expressing synaptic plasticity. J Neurosci 26(21):5767–5776
Fujiwara T et al (2016) Unusual social behavior in HPC-1/syntaxin1A knockout mice is caused by disruption of the oxytocinergic neural system. J Neurochem 138(1):117–123
Rovaris DL et al (2014) Should we keep on? Looking into pharmacogenomics of ADHD in adulthood from a different perspective. Pharmacogenomics 15(10):1365–1381
Barakauskas VE et al (2010) A novel mechanism and treatment target for presynaptic abnormalities in specific striatal regions in schizophrenia. Neuropsychopharmacology 35(5):1226–1238
da Silva BS et al (2017) Exocytosis-related genes and response to methylphenidate treatment in adults with ADHD. Mol Psychiatry 23(6):1446–1452
Haase J et al (2001) Regulation of the serotonin transporter by interacting proteins. Biochem Soc Trans 29(Pt 6):722–728
Quist JF, Kennedy JL (2001) Genetics of childhood disorders: XXIII. ADHD, Part 7: the serotonin system. J Am Acad Child Adolesc Psychiatry 40(2):253–256
Fujiwara T et al (2010) HPC-1/syntaxin 1A gene knockout mice show abnormal behavior possibly related to a disruption in 5-HTergic systems. Eur J Neurosci 32(1):99–107
Yu YX et al (2006) Syntaxin 1A promotes the endocytic sorting of EAAC1 leading to inhibition of glutamate transport. J Cell Sci 119(Pt 18):3776–3787
Acknowledgements
We are sincerely grateful to Dr. Jun Lin from Wuhan Medical and Health Center for Women and Children, and Dr. Yan Zhong from Children's Hospital of Hunan province for their support to our study. This study was supported partially by the National Natural Science Foundation of China (81773456), and the Fundamental Research Funds for the Central Universities, HUST (2016 YXMS218) to Dr. Jing Wu.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interests.
Informed consent
Written informed consent was obtained from all participants. The Ethics Committees of Tongji Medical College of Huazhong University of Science and Technology approved this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, M., Gu, X., Huang, X. et al. STX1A gene variations contribute to the susceptibility of children attention-deficit/hyperactivity disorder: a case–control association study. Eur Arch Psychiatry Clin Neurosci 269, 689–699 (2019). https://doi.org/10.1007/s00406-019-01010-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-019-01010-3