Abstract
The corpus callosum has become a key area of interest for researchers in severe mental illness. Disruptions in fractional anisotropy in the callosum have been reported in schizophrenia and major depressive disorder. No change has been reported in oligodendrocyte density and overall size of the callosum in either illness, suggesting that gross morphology is unchanged, but subtler organisational disruption may exist within this structure. Using high-resolution oil immersion microscopy, we examined the cross-sectional area of the nerve fibre and the axonal myelin sheath; and using standard high-resolution light microscopy, we measured the density of myelinated axons. These measurements were made in the splenium of the corpus callosum. Measures were taken in the sagittal plane in the callosal splenium to contrast with the previous similar examination of the callosal genu. Cases of major depressive disorder had significantly decreased mean myelin cross-sectional area (p = 0.014) per axon in the splenium than in controls or schizophrenia groups. There was no significant change in the density of myelinated axons. The results suggest a clear decrease of myelin in the axons of the callosal splenium in MDD, although this type of neuropathological study is unable to clarify whether this is caused by changes during life or has a developmental origin. In contrast with increased myelin in the callosal genu, this result suggests a longitudinal change in callosal myelination in major depressive disorder not present in normal or schizophrenic brains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The corpus callosum (CC) is the largest white matter (WM) structure in the CNS, running along the midline of the brain and uniting the two cerebral hemispheres. The thickest and most posterior portion of the CC is termed the splenium of the corpus callosum (spCC), which curves ventrally and continues anteriorly. It overlaps with the tela choroidea of the third ventricle and the posterior part of the dorsal midbrain. Axonal fibres comprising the CC join the cerebral hemispheres by way of homotopic and heterotopic connections, with a majority of commissural fibres uniting symmetrical areas of the hemispheres, although a proportion terminate in different contralateral regions from which they emanate. The spCC primarily connects regions of the parietal and occipital lobes [1], although the anterior part contains projections from medial temporal association areas [2].
The splenium itself is roughly composed of two primary fibre groups, composed of primarily reciprocal fibres from the temporal association and parietal association areas [2,3,4,5], although animal research has suggested that splenium fibres play a role regulating thalamocortical connections to the visual cortex [5, 6].
A significant callosal thinning was restricted to the genu in the early onset patients, but patients with late-onset depression exhibited a significant callosal thinning in both genu and splenium relative to controls. The splenium of the corpus callosum was also significantly thinner in subjects with late- vs early onset depression. Genu and splenium midsagittal areas significantly correlated with memory and attention functioning among late-onset depressed patients, but not early onset depressed patients or controls. Circumscribed structural alterations in callosal morphology may distinguish late- from early onset depression in the elderly [7].
The splenium of the CC connects the posterior cortices with fibres varying in sizes from thin late-myelinating axons in the anterior part, predominantly connecting parietal and temporal areas, to thick early‘myelinating fibres in the posterior part, linking primary and secondary visual areas. Splenium function in the human brain defined by the specialization of the area and implemented via excitation and/or suppression of the contralateral homotopic and heterotopic areas at the same level or different level of visual hierarchy. These mechanisms are facilitated by interhemispheric synchronization of oscillatory activity, also supported by the splenium. In the early childhood, myelination of the splenium correlates with the formation of interhemispheric excitatory influences in the extrastriate areas, whilst increasing inhibitory effects in the striate cortex are linked to the local inhibitory circuitry. Reshaping interactions between interhemispherically distributed networks are thought to lead to a reduction of metabolic and structural redundancy in the human brain [8].
Anatomical abnormalities in the CC in depression have been reported in diffusion weighted imaging [9, 10] and magnetisation transfer imaging [11,12,13], as MRI signal intensity can be used as a putative index of corpus callosum myelination. Bipolar patients had lower corpus callosum signal intensity for all callosal subregions (genu, anterior and posterior body, isthmus, splenium) than controls, whilst a whole brain myelin decrease has been reported in MDD, focused particularly in the dlPFC and NAcc [14]. Both SZ and MDD have been demonstrated to have changes in the CC. In vivo diffusion tensor imaging (DTI) studies have shown decreased fractional anisotropy (FA) in and across the WM in SZ [15], specifically in the spCC but not the callosal genu (gCC) [16,17,18]. First episode SZ has been reported to not be changed in callosal FA [19], but with decreased size of the gCC, body, and spCC, in contrast to other findings of overall increased thickness in chronic SZ [20,21,22]. DTI studies have shown that treatment-resistant MDD has lowered callosal FA, which is not related to illness severity [23, 24], and that FA was significantly decreased in the gCC in MDD, as well as bipolar disorder [25, 26]. Neuropathological studies of the callosum have shown no change in axon density, oligodendrocyte density, or overall gliosis in MDD or SZ, although, previously, we and others have reported an increase in axonal myelin in the callosal genu (gCC) in MDD [27,28,29,30].
Myelin-related and oligodendroglial genes exhibit decreased transcriptional activity in post-mortem tissue studies, suggesting oligodendroglial dysfunction in SZ. Microarrays have shown a significant reduction in expression of myelination-related genes MAG, PLLP, PLP1, gelsolin, and ERBB3 in SZ [31]. MAG regulates myelination and the development of myelin-producing cells in the CNS [32], whilst ERBB3 encodes neuregulin receptor Her3 that is required for oligodendrocyte survival and differentiation, and gelsolin is involved in stabilising the actin cytoskeleton of axons [33]. The hippocampus and cingulate cortex, both strongly related to the structure and function of the fornix and gCC, caudate, putamen, and multiple cortical regions also show decreased expression of myelin-related genes and oligodendrocyte markers in SZ [34, 35].
Oligodendrocyte-gene expression is not altered in MDD in thalamic or striatal regions, but two astrocyte markers, GFAP and ALDH1L1, are increased across thalamic and striatal regions in both SZ and MDD. In contrast microarray examination of temporal lobe, samples from MDD show 17 genes with altered expression, 16 of which are involved in myelin formation of oligodendrocyte regulation [32, 36]. Meta-analyses has shown decreased expression of MAG, ERBB, TF, PLP1, MOBP, and MOG across multiple brain regions, reviewed in Sokolov [37].
Protein studies suggest that many signalling proteins, such as ephrin B and ciliary neurotrophic factor, and those associated with cell growth and maintenance such as neurofilaments and tubulins, cell communication and signalling, and oligodendrocyte function such as myelin basic protein and myelin-oligodendrocyte glycoprotein are differentially phosphorylated and expressed in the CC in SZ [38, 39]. These results overlap with other post-mortem studies on proteins in SZ and suggest alternative significance of these changes in SZ as well as MDD.
This study aims to examine the myelin thickness, axonal internal size, and density of myelinated axons by means of novel high-resolution light microscopy using an optimised histological stain in the spCC in well-characterised cohorts of SZ, MDD, and controls using precisely optimised histological staining and high-resolution oil immersion microscopy.
Methods
Cases
Sagittal blocks containing the spCC were obtained from the Corsellis Tissue Bank in West London Mental Health Trust UK, according to strict inclusion/exclusion criteria. All the brains come from the Corsellis Brain Collection, consisting of over 6500 brains collected during the period 1952–1997. The collection consists of brains of individuals whose death was reported to the coroner’s court reported by Professor JAN Corsellis and retained for further research. The majority of samples come from the county of Essex, but a smaller number came from national referrals [40]. Medical notes were reviewed by a consultant psychiatrist and patients were selected on fulfillment of the WHO International Classification of Disease 10th edition (ICD-10) criteria, for SZ and MDD. For robustness of diagnosis of the brains of the three diagnostic groups, all suffered from chronic psychiatric illness or no recorded incidence of such in the case of controls. Assessment for any neurodegenerative, neurovascular, or infectious pathology, including Parkinson’s disease, was undertaken by a consultant pathologist and affected patients excluded. Patients with any recorded history of alcohol or drug abuse were also excluded. In SZ selection, the presence of first-rank symptoms was a necessity, and cases with onset older than 30 were excluded. Age- and post-mortem interval (PMI)-matched controls were selected as far as possible within the selection criteria. 59 cases were examined overall. 20 controls, 23 SZ, and 16 MDD, were included in the study (individual details in Table 1 and group means in Table 2).
Dissection and tissue processing
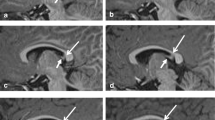
The spCC was dissected in the sagittal plane from selected cases (Fig. 1) 10% formalin (4% formaldehyde v/v) and stored in 10% formalin (4% formaldehyde v/v), before tissue processing. Blocks were processed using a Leica C300 tissue processor on a routine 12 h programme. Blocks were paraffin-embedded, sectioned at 10 µm thickness, and mounted on 25 × 75 mm electrostatic glass slides. For each case, 3 × 10 µm slides at 100 µm intervals per region were used for staining. Cases were blinded by an independent investigator before measurement.
Histological staining
One 10 µm section from each region was stained with hematoxylin and eosin (H&E). The sections were incubated in xylene for 15 min, then hydrated in 100, 90, and 70% ethanol (EtOH) and distilled water (dH2O) for 2 min each. Sections were immersed in 50% Mayer’s hematoxylin for 5 min before being rinsed in tap water (tH2O) and differentiated in 70% EtOH/1% hydrochloric acid (HCl) by immersing the sections for 3 × 1 s. Sections were incubated in 1% eosin for 5 min, rinsed in tH2O, dehydrated through serial alcohols, immersed in xylene, and mounted using DPX.
Two 10 µm sections per region were stained with Luxol Fast Blue (LFB). These were de-waxed in xylene and immersed in two washes of 100% EtOH for 2 min each time. Sections were immersed in 1% LFB/methanol solution overnight in a 60 °C oven. Sections were rehydrated in 100, 90, and 70% EtOH and rinsed in tH2O until water ran colourless. Sections were differentiated in saturated lithium carbonate solution, dehydrated through serial alcohols, immersed in xylene, and mounted using DPX.
All cases were blinded by an independent investigator before image capture and measurement.
Image capture
Sections stained with H&E were used for neuroanatomical guidance; adjacent LFB-stained slides were used for axonal measures. All assay and measurement images were captured at 2096 × 1536 resolution using an Olympus Vanox AHBT 3 microscope with a Q Imaging Micropublisher RTV 3.3 camera and analysed using Image-Pro Plus 5.1 software (Media Cybernetics, US). Density measurements were taken at 400× total magnification, whilst axonal architecture measures were taken at 1000× under oil immersion.
Microscopy
LFB-stained sections from the splenium were used for all measures with H&E sections as an anatomical guide. Images for axon measures were all taken with oil immersion at 1000× magnification. Three images per slide were taken under the criterion that they contain more than three axons, so that enough measures could be obtained. Axons had to exhibit clearly defined myelin rings that were darker than both the inside of the axon and the surroundings, so that they could be traced accurately and without difficulty. A total of eight axons were measured from each region of each LFB-slide, a number determined previously to be the optimum number of neuroarchitectural measures to make for maximum efficiency [29, 41, 42]. Images were taken at 1000× magnification from the exact centre of each region on each of the two LFB-slides per case for density measures (as shown in Fig. 1).
Measures
Measures were taken according to the results of calibration assays previously described [29]. All visible transverse axons in each image were tagged and then randomly selected using a random number generator. In each of the three sampled regions from each of the slides, regions shown in grey in Fig. 1, a total of randomly selected eight axons were measured for myelin thickness and internal axon diameter as described by the assay protocol.
For each case, there were 20 measurements per axon, 8 axons per region, 3 regions per slide, and 2 slides per case. This gave a total of 960 measures on 48 randomly selected axons per case. All measures of each type were pooled before analysis.
Cross-sectional area (CSA) measures of internal axon diameter and myelin area were measured on static images taken at 1000× as described above. Area was calculated by appropriate calibration the image to the magnification, and by tracing along the inner and outer edges of the sheath the area of the myelin ring. The calibrated computer software gave CSA in µm2.
Data analysis
Slides were unblinded after all measures had been collected and data collected between different slides for the same region and case was merged for analysis. All statistical analyses were performed using Windows SPSS 16.0. The four measures of mean myelin thickness, myelin CSA, mean axon thickness, and axon CSA were examined independently in the spCC. Each of the measures was analysed using univariate analysis with general linear model (GLM).
The primary comparison was changes between diagnostic groups, with diagnosis along with sex and age examined in the GLM as interacting variables. In addition, in each analysis using the GLM potential confounders such as fixation period, incidence of suicide and PMI were examined as confounding variables. As a secondary analysis following significant findings, direct tests between single groups were performed using t tests.
Ethics
This project was conducted under ethical permission granted by the London south west local ethics committee reference WL/02/12 (2002), and amendment WL/02/12/AM01, granted by the Ealing and WLMHT local research ethics committee (2006).
Results
The SZ group was significantly older than the MDD group (p = 0.012), and in formalin for a longer period (p = 0.021; see Table 2). There was a significant interaction in GLM analysis between age and myelin CSA (p = 0.038), although regression analysis showed no direct relationship between age and myelin CSA in the spCC (p = 0.377, r2 = 0.00052). There was no significant effect of age, sex, PMI, or formalin-fixation period interacting with any other confounding variable or upon myelin thickness, internal axonal diameter, or axon density in the splenium. There was no effect of the incidence of anti-psychotic treatment (p = 0.836) or chlorpromazine-equivalent units with presented results (p = 0.600), and no effect of anti-depressant treatment (p = 0.644).
The incidence of suicide is unevenly distributed between the groups (controls n = 0, SZ n = 2 confirmed, it was unclear whether drowning in case 29 was accidental, MDD n = 9). Within the groups, there was no interaction of suicide on the measurements (SZ, p = 0.249; MDD, p = 0.603).
MDD brains showed significantly smaller myelin CSA (control: 14.4 ± 1.4 µm2, MDD: 9.2 ± 1.0 µm2, df8,46, 36% decrease, p = 0.015; Fcrit 8,46 = 2.15; Fig. 2) but not myelin thickness (control: 1.04 ± 0.05 µm, MDD: 0.97 ± 0.03 µm, df8,46,p = 0.413; Fcrit 8,46 = 2.15; Fig. 3) in the spCC compared to SZ and control.
There was no change in axonal internal CSA (control: 8.5 ± 1.3 µm2, MDD: 10.5 ± 1.3 µm2, df8,46,p = 411; Fcrit 8,46 = 2.15; Fig. 4) or internal axonal diameter in MDD compared to the other diagnostic groups (control: 3.0 ± 0.25 µm2, MDD: 3.1 ± 0.18 µm2, df8,46; p = 0.900; Fcrit 8,46 = 2.15; Fig. 5).
There was no significant change in the density of myelinated axons between diagnostic groups in the spCC (p = 0.182).
There was no change in the spCC in SZ between diagnostic groups in myelin CSA (spCC, p = 0.672), myelin thickness (spCC, p = 0.445), internal axonal CSA (spCC, p = 0.273), internal axonal diameter (spCC, p = 0.654), or in axon density (spCC, p = 0.806).
The results are summarised in Table 3.
Discussion
The results demonstrate a significant decrease in the myelin CSA of spCC axons in MDD, with no change observed in axon size or density. This change is the opposite effect observed in the gCC, suggesting an anterior–posterior change in axon structures along the callosum in MDD not present in SZ or controls.
Biological significance
The results presented could indicate in hemispheric connection via the spCC between the parietal or occipital lobes in MDD. The thinner myelin suggests slower neural transmission rates in MDD in the splenium, as healthy myelin coating of nerve axons is related to signal transmission speed, although as axons carry inhibitory signals as well as excitatory ones, this effect may not be clinically obvious. Whilst no structural changes in the parietal or occipital lobes have been reported in schizophrenia [43], an increase in occipital malformation where one lobe bends around the other has been reported in depression [44]. A neurodevelopmental disorder such as this could possibly affect the development of reciprocal connections through the splenium. Low GABA concentrations have also been reported in the occipital lobe in depression, possibly reflecting a functional change in this structure that may affect maintenance of the myelin on associated networks.
Whilst myelin thickness is determined by oligodendrocyte function, there is no direct evidence published to suggest decreased oligodendrocyte activity in this region. Decreases in myelin- and oligodendrocyte-gene expression have been reported in MDD [32, 36, 37], but functional gene network modelling has suggested that this may not indicate large-scale disruption of the gene networks, and, hence, physiological function, as many changes may be to compensate for by other as yet undiscovered alterations [45]. Such reported changes may compensate for one and it is unclear if disruption in a network directly relates to decrease physiological output. It is entirely possible that examination of these gene networks by mRNA methods alone without better understanding of protein synthesis, glial cell function, and mechanisms is providing an inaccurate picture of myelin synthesis in these illnesses. As similar gene expression changes have been reported in bipolar disorder [37], it would be useful to examine this in future studies to contrast both the SZ and MDD findings.
Methodology
The differences in the two groups in age, and PMI, fixation, though significant, were very small. The significance comes from the very tight selection of cases within these groups (SZ group mean age was only 3.3 years older than MDD group, Table 2). The SZ group was also in formalin fixation for longer than the MDD group, though this had no effect on the results. The previous studies on these disorders and this anatomical structure from the Corsellis collection have shown that formalin-fixation period does not affect neuropathological markers using histological staining [29, 42, 46, 47]. The lack of differences between the control and SZ groups in any measurement and the lack of significant interaction between confounding factors and results also suggest that any SZ and MDD difference is real and not an artifact of group variability.
It has been found that the nodes of Ranvier and the internodal sections of the axon increase in length in proportion to the diameter of the axon. In the CNS axon diameter varies from 0.2 to 20 µm and the myelin sheath is interrupted every 1–2 mm by a node of Ranvier approximately 2 µm in length. Our sections of 10, 100 µm apart, would not be affected by the size and distribution of the nodes. Increasing numbers of measurements of either myelin width or internal diameter past the optimum did not enhance the accuracy of the results. By measuring on two slides 100 µm apart, we negate any interacting effect of nodes.
When quantifying the width of myelin of irregular axons, the most precise data were obtained by measuring the width perpendicular to the intersection points. The lines provided by the asterisk were too long in many cases as they traversed the sheath at very shallow angles and, therefore, did not reflect the sheath width accurately. The use of a grid mask facilitated the unbiased selection of fixed points at which the width measurements were obtained, even when looking at irregular axons [29].
Limitations
Given the inherent difficulties in post-mortem studies, and the subsequent heterogeneity of results seen in many brain regions in neuropsychiatric studies, this investigation certainly should be repeated by independent investigators using brain tissue sourced from a different bank.
First, we have the issue of case selection, so commonly a difficult issue in neuropathology. Whilst the cases were extensively reviewed for inclusion by modern ICD-10 criteria with defined psychotic symptomatology and tightly age-matched, there was a significant sex bias between the groups. Although, statistically, this had no effect, ideally, this should be repeated with a greater balance of sexes to rule out any masked confounders.
Second, we used two methods to examine the amount of myelin at the level of measurement, thickness, and CSA. It could be reasonably said that these are two ways of measuring the same thing. However, the significant effect was only observed in CSA. This is likely to be due to the 2D effect measured by CSA being larger than the 1D effect measured by a simple line measure, rather than any actual significant biological difference. We anticipate that the reader will be able to make this distinction when drawing their conclusions from these results though.
Future work
A repeat investigation of splenium axons is necessary as changes in FA have been noted in this structure [16]. It may be of interest to study the unmyelinated axons of the CC using antibodies against neurofilament to carry out immunofluorescence on these structures. The relative numbers of both types of axon in SZ, MDD, and control patients may also be significant as, perhaps, loss of one type is more pronounced than the other.
References
Nolte J (2009) The human brain: an introduction to its functional anatomy, 6th edn. Mosby/Elsevier, Philadelphia, US
Hofer S, Frahm J (2006) Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 32(3):989–994
Aboitiz F et al (1992) Fiber composition of the human corpus callosum. Brain Res 598(1–2):143–153
Dougherty RF et al (2005) Occipital–callosal pathways in children: validation and atlas development. Ann N Y Acad Sci 1064:98–112
Saenz M, Fine I (2010) Topographic organization of V1 projections through the corpus callosum in humans. Neuroimage 52(4):1224–1229
Makarov VA et al (2008) Stimulus-dependent interaction between the visual areas 17 and 18 of the 2 hemispheres of the ferret (Mustela putorius). Cereb Cortex 18(8):1951–1960
Ballmaier M et al (2008) Mapping callosal morphology in early- and late-onset elderly depression: an index of distinct changes in cortical connectivity. Neuropsychopharmacology 33(7):1528–1536
Knyazeva MG (2013) Splenium of corpus callosum: patterns of interhemispheric interaction in children and adults. Neural Plast 2013:639430
Wise T et al (2016) Voxel-based meta-analytical evidence of structural disconnectivity in major depression and bipolar disorder. Biol Psychiatry 79(4):293–302
Beasley CL et al (2005) Evidence for altered neuronal organisation within the planum temporale in major psychiatric disorders. Schizophr Res 73(1):69–78
Wyckoff N et al (2003) Magnetization transfer imaging and magnetic resonance spectroscopy of normal-appearing white matter in late-life major depression. J Magn Reson Imaging 18(5):537–543
Kumar A et al (2004) Biophysical changes in normal-appearing white matter and subcortical nuclei in late-life major depression detected using magnetization transfer. Psychiatry Res 130(2):131–140
Zhang TJ et al (2009) Magnetization transfer imaging reveals the brain deficit in patients with treatment-refractory depression. J Affect Disord 117(3):157–161
Sacchet MD, Gotlib IH (2017) Myelination of the brain in major depressive disorder: an in vivo quantitative magnetic resonance imaging study. Sci Rep 7(1):2200
Davis KL et al (2003) White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry 60(5):443–456
Foong J et al (2000) Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry 68(2):242–244
Abdul-Rahman MF, Qiu A, Sim K (2011) Regionally specific white matter disruptions of fornix and cingulum in schizophrenia. PLoS One 6(4):e18652
Bora E et al (2011) Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res 127(1–3):46–57
Price G et al (2005) The corpus callosum in first episode schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry 76(4):585–587
Bigelow L, Rosenthal R (1972) Schizophrenia and the corpus callosum. Lancet 1(7752):694
Bigelow LB, Nasrallah HA, Rauscher FP (1983) Corpus callosum thickness in chronic schizophrenia. Br J Psychiatry 142:284–287
Keshavan MS et al (2002) Abnormalities of the corpus callosum in first episode, treatment naive schizophrenia. J Neurol Neurosurg Psychiatry 72(6):757–760
Guo WB et al (2012) Altered white matter integrity of forebrain in treatment-resistant depression: a diffusion tensor imaging study with tract-based spatial statistics. Prog Neuropsychopharmacol Biol Psychiatry 38(2):201–206
Hoogenboom WS et al (2014) Limbic system white matter microstructure and long-term treatment outcome in major depressive disorder: a diffusion tensor imaging study using legacy data. World J Biol Psychiatry 15(2):122–134
Carrasco JL et al (2012) Microstructural white matter damage at orbitofrontal areas in borderline personality disorder. J Affect Disord 139(2):149–153
Xu K et al (2013) Impaired interhemispheric connectivity in medication-naive patients with major depressive disorder. J Psychiatry Neurosci 38(1):43–48
Nasrallah HA (1982) Neuropathology of the corpus callosum in schizophrenia. Br J Psychiatry 141:99–100
Williams MR et al (2013) Astrocyte decrease in the subgenual cingulate and callosal genu in schizophrenia. Eur Arch Psychiatry Clin Neurosci 263(1):41–52
Williams MR, Sharma P, Fung KL, Pearce RKB, Hirsch SR, Maier M (2015) Axonal myelin increase in the callosal genu in depression but not schizophrenia. Psychol Med 25:1–11
Ongur D, Drevets WC, Price JL (1998) Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA 95(22):13290–13295
Aston C, Jiang L, Sokolov BP (2004) Microarray analysis of postmortem temporal cortex from patients with schizophrenia. J Neurosci Res 77(6):858–866
Aston C, Jiang L, Sokolov BP (2005) Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol Psychiatry 10(3):309–322
Hakak Y et al (2001) Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA 98(8):4746–4751
Hof PR et al (2002) Molecular and cellular evidence for an oligodendrocyte abnormality in schizophrenia. Neurochem Res 27(10):1193–1200
Katsel P, Davis KL, Haroutunian V (2005) Variations in myelin and oligodendrocyte-related gene expression across multiple brain regions in schizophrenia: a gene ontology study. Schizophr Res 79(2–3):157–173
Barley K, Dracheva S, Byne W (2009) Subcortical oligodendrocyte- and astrocyte-associated gene expression in subjects with schizophrenia, major depression and bipolar disorder. Schizophr Res 112(1–3):54–64
Sokolov BP (2007) Oligodendroglial abnormalities in schizophrenia, mood disorders and substance abuse. Comorbidity, shared traits, or molecular phenocopies? Int J Neuropsychopharmacol 10(4):547–555
Saia-Cereda VM et al (2016) Differential proteome and phosphoproteome may impact cell signaling in the corpus callosum of schizophrenia patients. Schizophr Res 177(1–3):70–77
Saia-Cereda VM et al (2015) Proteomics of the corpus callosum unravel pivotal players in the dysfunction of cell signaling, structure, and myelination in schizophrenia brains. Eur Arch Psychiatry Clin Neurosci 265(7):601–612
Kasper BS et al (2010) Neuropathology of epilepsy and psychosis: the contributions of J.A.N. Corsellis. Brain 133(Pt 12):3795–3805
Williams MR et al (2013) Neuropathological changes in the nucleus basalis in schizophrenia. Eur Arch Psychiatry Clin Neurosci 263(6):485–495
Williams MR et al (2014) Neuropathological changes in the substantia nigra in schizophrenia but not depression. Eur Arch Psychiatry Clin Neurosci 264(4):285–296
Falkai P et al (2004) Shape changes in prefrontal, but not parieto-occipital regions: brains of schizophrenic patients come closer to a circle in coronal and sagittal view. Psychiatry Res 132(3):261–271
Maller JJ et al (2014) Occipital bending in depression. Brain 137(Pt 6):1830–1837
Gaiteri C, Sibille E (2011) Differentially expressed genes in major depression reside on the periphery of resilient gene coexpression networks. Front Neurosci 5:95
MR W et al (2012) The subgenual cingulate gyrus exhibits lower rates of bifurcation in schizophrenia than in controls, bipolar disorder and depression. Open J Psychiatry 2(4):253–257
Howes OD et al (2013) Midbrain dopamine function in schizophrenia and depression: a post-mortem and positron emission tomographic imaging study. Brain 136(Pt 11):3242–3251
Acknowledgements
The authors wish to thank Prof. Bishan Radotra for neuropathological advice and to Dr. Rabiah Chaudhry and Dr. Rachel March for help with dissection and Prof. Federico Turkheimer for statistical advice. The tissue was obtained from the Corsellis Brain Collection in 2008, supported by the Starr foundation and supported by funding from the MRC-UK PET Methodology Programme Grant G1100809/1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Williams, M.R., Sharma, P., Macdonald, C. et al. Axonal myelin decrease in the splenium in major depressive disorder. Eur Arch Psychiatry Clin Neurosci 269, 387–395 (2019). https://doi.org/10.1007/s00406-018-0904-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-018-0904-4