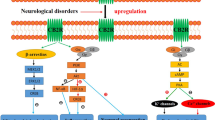
Abstract
Clinical and neurobiological findings have reported the involvement of endocannabinoid signaling in the pathophysiology of schizophrenia. This system modulates dopaminergic and glutamatergic neurotransmission that is associated with positive, negative, and cognitive symptoms of schizophrenia. Despite neurotransmitter impairments, increasing evidence points to a role of glial cells in schizophrenia pathobiology. Glial cells encompass three main groups: oligodendrocytes, microglia, and astrocytes. These cells promote several neurobiological functions, such as myelination of axons, metabolic and structural support, and immune response in the central nervous system. Impairments in glial cells lead to disruptions in communication and in the homeostasis of neurons that play role in pathobiology of disorders such as schizophrenia. Therefore, data suggest that glial cells may be a potential pharmacological tool to treat schizophrenia and other brain disorders. In this regard, glial cells express cannabinoid receptors and synthesize endocannabinoids, and cannabinoid drugs affect some functions of these cells that can be implicated in schizophrenia pathobiology. Thus, the aim of this review is to provide data about the glial changes observed in schizophrenia, and how cannabinoids could modulate these alterations.

Similar content being viewed by others
Abbreviations
- ∆9-THC:
-
Delta-9-tetrahydrocannabinol
- 2-AG:
-
2-Arachidonoylglycerol
- AEA:
-
Anandamide
- CB1:
-
Type 1 cannabinoid receptor
- CB2:
-
Type 2 cannabinoid receptor
- CNR1:
-
Cannabinoid receptor type-1 gene
- CNR2:
-
Cannabinoid receptor type-2 gene
- COX:
-
Cyclooxygenase
- DAGLα:
-
Diacylglycerol lipase alpha
- DAGLβ:
-
Diacylglycerol lipase beta
- DISC-1:
-
Disrupted in schizophrenia-1
- FAAH:
-
Fatty acid amide hydrolase
- GFAP:
-
Glial fibrillary acid protein
- GLAST:
-
Glutamate aspartate transporter
- GLT-1:
-
Astrocytic glutamate transporter-1
- GPR55:
-
G protein-coupled receptor 55
- IL-1:
-
Interleukin 1
- IL-6:
-
Interleukin 6
- KO:
-
Knockout
- LPS:
-
Lipopolysaccharide
- MAGL:
-
Monoacylglycerol lipase
- NAPE:
-
N-Acyl-phosphatidylethanolamine-phospholipase
- NMDA:
-
N-Methyl-d-aspartate
- OPCs:
-
Oligodendrocyte precursor cells
- PPARγ:
-
Peroxisome proliferator-activated receptor
- TNF-α:
-
Tumor necrosis factor alpha
- TRPV1:
-
Transient receptor potential vanilloid 1
References
Owen MJ, Sawa A, Mortensen PB (2016) Schizophrenia. Lancet 388(10039):86–97
Howes OD, Kapur S (2009) The Dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull 35(3):549–562
Kantrowitz J, Javitt D (2012) Glutamatergic transmission in schizophrenia: from basic research to clinical practice. Curr Opin Psychiatry 25(2):96–102
Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M (2009) Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol 19:220–230
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140(6):918–934
van Kesteren CF et al (2017) Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry 7(3):e1075
Bernstein HG, Steiner J, Guest PC, Dobrowolny H, Bogerts B (2015) Glial cells as key players in schizophrenia pathology: recent insights and concepts of therapy. Schizophr Res 161(1):4–18
Fernandez-Espejo E, Viveros MP, Nunez L, Ellenbroek BA, Rodriguez De Fonseca, F (2009) Role of cannabis and endocannabinoids in the genesis of schizophrenia. Psychopharmacology (Berlin) 206:531–549
Stella N (2009) Endocannabinoid signaling in microglial cells. Neuropharmacology 56(1):244–253
Walter L, Stella N (2003) Endothelin-1 increases 2-arachidonoyl glycerol (2-AG) production in astrocytes. Glia 44(1):85–90
Mechoulam R, Parker LA (2013) The endocannabinoid system and the brain. Annu Rev Psychol 64:21–47
Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC (1988) Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34:605–613
Devane WA et al (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949
Bisogno T et al (2003) Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signalling in the brain. J Cell Biol 163:463–468
Piomelli D (2003) The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4(11):873–884
Pertwee RG (2006) The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes (Lond) 30(1):S13-8
Munro S, Thomas KL, Abu-ShaarM (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365(6441):61–65
Brusco A, Tagliaferro P, Saez T, Onaivi ES (2008) Postsynaptic localization of CB2 cannabinoid receptors in the rat hippocampus. Synapse 62:944–949
Onaivi ES, Ishiguro H, Gu S, Liu QR (2012) CNS effects of CB2 cannabinoid receptors: beyond neuro-immuno-cannabinoid activity. J Psychopharmacol 26(1):92–103
Nevalainen T, Irving AJ (2010) GPR55, a lysophosphatidylinositol receptor with cannabinoid sensitivity? Curr Top Med Chem 10(8):799–813
Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, Moriello AS et al (2001) Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 134:845–852
Zygmunt PM et al (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400(6743):452–457
De Petrocellis L, Cascio MG, Di Marzo V (2004) The endocannabinoid system: a general view and latest additions. Br J Pharmacol 141:765–774
Di Marzo V, De Petrocellis L (2012) Why do cannabinoid receptors have more than one endogenous ligand? Philos Trans R Soc Lond B Biol Sci 367(1607):3216–3228
Wilson RI, Nicoll RA (2002) Endocannabinoid signaling in the brain. Science 296:678–682
Bacci A, Huguenard JR, Prince DA (2004) Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature 431(7006):312–316
Bénard G et al (2012) Mitochondrial CB(1) receptors regulate neuronal energy metabolism. Nat Neurosci 15:558–564
Hebert-Chatelain E et al (2014) Cannabinoid control of brain bioenergetics: exploring the subcellular localization of the CB1 receptor. Mol Metab 3(4):495–504
Scheller A, Kirchhoff F (2016) Endocannabinoids and heterogeneity of glial cells in brain function. Front Integr Neurosci 10:24. https://doi.org/10.3389/fnint.2016.00024.eCollection2016
Bersani G, Orlandi V, Kotzalidis GD, Pancheri P (2002) Cannabis and schizophrenia: impact on onset, course, psychopathology and outcomes. Eur Arch Psychiatry Clin Neurosci 252(2):86–92
Barnett JH, Werners U, Secher SM (2007) Substance use in a population based clinic sample of people with first-episode psychosis. Br J Psychiatry 190:515–520
Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE (2002) Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ 325(7374):1212–1213
Henquet C, Krabbendam L, Spauwen J, Kaplan C, Lieb R, Wittchen HU, van Os J (2005) Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. BMJ 330:11
Ksir C, Hart CL (2016) Cannabis and psychosis: a critical overview of the relationship. Curr Psychiatry Rep.18(2):12. https://doi.org/10.1007/s11920-015-0657-y
Leweke FM, Giuffrida A, Wurster U, Emrich HM, Piomelli D (1999) Elevated endogenous cannabinoids in schizophrenia. Neuroreport 10:1665–1669
Reuter AR et al (2016) Association of anandamide with altered binocular depth inversion illusion in schizophrenia. World J Biol Psychiatry 11:1–6 (Epub ahead of print)
DeMarchi N et al (2003) Endocannabinoid signalling in the blood of patients with schizophrenia. Lipids Health Dis 2:5
Potvin S, Stip E, Lipp O, Roy MA, Demers MF, Bouchard RH, Gendron A (2008) Anhedonia and social adaptation predict substance abuse evolution in dual diagnosis schizophrenia. Am J Drug Alcohol Abuse 34(1):75–82
Ceccarini J et al (2010) In vivo PET imaging of cerebral type 1 cannabinoid receptor availability in patients with schizophrenia. Schizophr Res 117:70
Dalton VS, Long LE, Weickert CS, Zavitsanou K (2011) Paranoid schizophrenia is characterized by increased CB1 receptor binding in the dorsolateral prefrontal cortex. Neuropsychopharmacology 36:1620–1630
Dean B, Sundram S, Bradbury R, Scarr E, Copolov D (2001) Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience 103:9–15
Eggan SM, Stoyak SR, Verrico CD, Lewis DA (2010) Cannabinoid CB1 receptor immunoreactivity in the prefrontal cortex: comparison of schizophrenia and major depressive disorder. Neuropsychopharmacology 35(10):2060–2071
Urigüen L et al (2009) Immunodensity and mRNA expression of A2A adenosine, D2 dopamine, and CB1 cannabinoid receptors in postmortem frontal cortex of subjects with schizophrenia: effect of antipsychotic treatment. Psychopharmacol Berl 206(2):313e324
Wong DF et al (2010) Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. Neuroimage 52:1505–1513
Zavitsanou K, Garrick T, Huang XF (2004) Selective antagonist [3H]SR141716A binding to cannabinoid CB1 receptors is increased in the anterior cingulate cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 28:355–360
Delis F, Rosko L, Shroff A, Leonard KE, Thanos PK (2017) Oral haloperidol or olanzapine intake produces distinct and region-specific increase in cannabinoid receptor levels that is prevented by high fat diet. Prog Neuropsychopharmacol Biol Psychiatry 79(Pt B):268–280. https://doi.org/10.1016/j.pnpbp.2017.06.005
Chavarría-Siles I et al (2008) Cannabinoid receptor 1 gene (CNR1) and susceptibility to a quantitative phenotype for hebephrenic schizophrenia. Am J Med Genet B Neuropsychiatr Genet 147(3):279–284
Martínez-Gras I et al (2006) (AAT)n repeat in the cannabinoid receptor gene, CNR1: association with schizophrenia in a Spanish population. Eur Arch Psychiatry Clin Neurosci 256(7):437–441
Suárez-Pinilla P et al (2015) Brain structural and clinical changes after first episode psychosis: Focus on cannabinoid receptor 1 polymorphisms. Psychiatry Res 233(2):112–119. https://doi.org/10.1016/j.pscychresns.2015.05.005
Ujike H et al (2002) CNR1, central cannabinoid receptor gene, associated with susceptibility to hebephrenic schizophrenia. Mol Psychiatry 7(5):515–518
Ishiguro H et al (2010) Brain cannabinoid CB2 receptor in schizophrenia. Biol Psychiatry 67:974–982
Ho BC, Wassink TH, Ziebell S, Andreasen NC (2011) Cannabinoid receptor 1 gene polymorphisms and marijuana misuse interactions on white matter and cognitive deficits in schizophrenia. Schizophr Res 128:66–75
Almeida V et al (2014) Effects of cannabinoid and vanilloid drugs on positive and negative-like symptoms on an animal model of schizophrenia: the SHR strain. Schizophr Res 153(1–3):150–159
Levin R et al (2014) Effects of cannabinoid drugs on the deficit of prepulse inhibition of startle in an animal model of schizophrenia: the SHR strain. Front Pharmacol 6:5:10
Malone DT, Taylor DA (2006) The effect of delta9-tetrahydrocannabinol on sensorimotor gating in socially isolated rats. Behav Brain Res 166(1):101–109
Schneider M, Koch M (2002) The cannabinoid agonist WIN 55,212-2 reduces sensorimotor gating and recognition memory in rats. Behav Pharmacol 13:29–37
Wegener N, Kuhnert S, Thuns A, Roese R, Koch M (2008) Effects of acute systemic and intra-cerebral stimulation of cannabinoid receptors on sensorimotor gating, locomotion and spatial memory in rats. Psychopharmacology (Berlin) 198:375–385
Ballmaier M et al (2007) Cannabinoid receptor antagonists counteract sensorimotor gating deficits in the phencyclidine model of psychosis. Neuropsychopharmacology 32(10):2098–2107
Levin R et al (2012) Antipsychotic profile of cannabidiol and rimonabant in an animal model of emotional context processing in schizophrenia. Curr Pharm Des 18(32):4960–4965
Campos AC et al (2017) Plastic and Neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front Pharmacol 8:269. https://doi.org/10.3389/fphar.2017.00269.eCollection2017
Peres FF et al (2016) Peripubertal treatment with cannabidiol prevents the emergence of psychosis in an animal model of schizophrenia. Schizophr Res 172(1–3):220–221
Zuardi AW, Morais SL, Guimarães FS, Mechoulam R (1995) Anti-psychotic effect of cannabidiol. J Clin Psychiatry 56:485–486
Leweke FM et al (2012) Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2:e94. https://doi.org/10.1038/tp.2012.15
McGuire P et al (2017) Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. https://doi.org/10.1176/appi.ajp.2017.17030325
Bora E et al (2011) Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res 127(1–3):46–57
Breier A et al (1992) Brain morphology and schizophrenia. A magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Arch Gen Psychiatry 49(12):921–926
Sigmundsson T et al (2001) Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry 158(2):234–243
Fujino J et al (2014) Impaired empathic abilities and reduced white matter integrity in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 48:117–123
Holleran L et al (2014) Altered interhemispheric and temporal lobe white matter microstructural organization in severe chronic schizophrenia. Neuropsychopharmacology 39(4):944–954
Cassoli JS et al (2015) Disturbed macro-connectivity in schizophrenia linked to oligodendrocyte dysfunction: from structural findings to molecules. NPJ Schizophr 1:15034
Falkai P et al (2016) Decreased oligodendrocyte and neuron number in anterior hippocampal areas and the entire hippocampus in schizophrenia: a stereological postmortem study. Schizophr Bull 42(Suppl 1):S4-S12. https://doi.org/10.1093/schbul/sbv157
Uranova NA, Vikhreva OV, Rachmanova VI, Orlovskaya DD (2011) Ultrastructural alterations of myelinated fibers and oligodendrocytes in the prefrontal cortex in schizophrenia: a postmortem morphometric study. Schizophr Res Treat. 325789. https://doi.org/10.1155/2011/325789
Vikhreva OV, Rakhmanova VI, Orlovskaya DD, Uranova NA (2016) Ultrastructural alterations of oligodendrocytes in prefrontal white matter in schizophrenia: a post-mortem morphometric study. Schizophr Res 177(1–3):28–36
Wang HN et al. (2015) Quetiapine ameliorates schizophrenia-like behaviors and protects myelin integrity in cuprizone intoxicated mice: the involvement of notch signaling pathway. Int J Neuropsychopharmacol. https://doi.org/10.1093/ijnp/pyv088
Cassoli JS et al (2016) Effect of MK-801 and clozapine on the proteome of cultured human oligodendrocytes. Front Cell Neurosci 10:52
Garver DL, Holcomb JA, Christensen JD (2008) Compromised myelin integrity during psychosis with repair during remission in drug-responding schizophrenia. Int J Neuropsychopharmacol 11:49–61
Kimoto S et al (2011) Olanzapine stimulates proliferation but inhibits differentiation in rat oligodendrocyte precursor cell cultures. Prog Neuropsychopharmacol Biol Psychiatry 35(8):1950–1956
Niu J et al (2010) Haloperidol promotes proliferation but inhibits differentiation in rat oligodendrocyte progenitor cell cultures. Biochem Cell Biol 88(4):611–620
Xu H, Yang HJ, Li XM (2014) Differential effects of antipsychotics on the development of rat oligodendrocyte precursor cells exposed to cuprizone. Eur Arch Psychiatry Clin Neurosci 264(2):121–129
Steiner J et al (2014) Clozapine promotes glycolysis and myelin lipid synthesis in cultured oligodendrocytes. Front Cell Neurosci 8:384
Molina-Holgado E et al (2002) Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. JNeurosci 22:9742–9753
Solbrig MV, Fan Y, Hermanowicz N, Morgese MG, Giuffrida A (2010) A synthetic cannabinoid agonist promotes oligodendrogliogenesis during viral encephalitis in rats. Exp Neurol 226:231–241
Sun J,et al (2013) WIN55, 212-2 promotes differentiation of oligodendrocyte precursor cells and improve remyelination through regulation of the phosphorylation level of the ERK 1/2 via cannabinoid receptor 1 after stroke-induced demyelination. Brain Res 1491:225–235
Gomez O et al (2011) Cannabinoid receptor agonists modulate oligodendrocyte differentiation by activating PI3K/Akt and the mammalian target of rapamycin (mTOR) pathways. Br J Pharmacol 163:1520–1532
Tomas-Roig J, Wirths O, Salinas-Riester G, Havemann-Reinecke U (2016) The cannabinoid CB1/CB2 agonist WIN55212.2 promotes oligodendrocyte differentiation in vitro and neuroprotection during the cuprizone-induced central nervous system demyelination. CNS Neurosci Ther 22(5):387–395
Mecha M et al (2012) Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress. Cell Death Dis 28(3):e331
Barron H, Hafizi S, Andreazza AC, Mizrahi R (2017) Neuroinflammation and oxidative stress in psychosis and psychosis risk. Int J Mol Sci 18(3):651
Steullet P et al (2016) Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: A “central hub” in schizophrenia pathophysiology? Schizophr Res 176(1):41–51
Rahimi A et al (2015) Interaction between the protective effects of cannabidiol and palmitoylethanolamide in experimental model of multiple sclerosis in C57BL/6 mice. Neuroscience 290:279–287
Mato S, Victoria Sanchez-Gomez M, Matute C (2010) Cannabidiol induces intracellular calcium elevation and cytotoxicity in oligodendrocytes. Glia 58:1739–1747
Blankman JL, Simon GM, Cravatt BF (2009) A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Phys Lipids 14:1347–1356
Panikashvili D et al (2001) An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature 413(6855):527–531
Chen R et al (2012) Monoacylglycerol lipase is a therapeutic target for Alzheimer’s disease. Cell Rep 2:1329–1339
Nomura DK et al (2011) Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 334:809–813
Piro JR et al (2012) A dysregulated endocannabinoid-eicosanoid network supports pathogenesis in a mouse model of Alzheimer’s disease. Cell Rep 1:617–623
Bernal-Chico A et al (2015) Blockade of monoacylglycerol lipase inhibits oligodendrocyte excitotoxicity and prevents demyelination in vivo. Glia 63(1):163–176
Gomez O et al (2010) The constitutive production of the endocannabinoid 2-arachidonoylglycerol participates in oligodendrocyte differentiation. Glia 58(16):1913–1927
Trépanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP (2016) Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry 21(8):1009–1026
Goudriaan A et al. (2014) Specific glial functions contribute to schizophrenia susceptibility. Schizophr Bull. 40(4):925–935
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119(1):7–35
Araque A, Parpura V, Sanzgiri RP, Haydon PG (1999) Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22:208–215
Araque A et al (2014) Gliotransmitters travel in time and space. Neuron 81(4):728–739
Perea G, Navarrete M, Araque A (2009) Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32:421–431
Bernardinelli Y et al (2014) Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr Biol 24:1679–1688
Perez-Alvarez A, Navarrete M, Covelo A, Martin ED, Araque A (2014) Structural and functional plasticity of astrocyte processes and dendritic spine interactions. J Neurosci 34:12738–12744
Ma TM et al (2013) Pathogenic disruption of DISC1-serine racemase binding elicits schizophrenia-like behavior via D-serine depletion. Mol Psychiatry 18:557–567
Xia M, Zhu S, Shevelkin A, Ross CA, Pletnikov M (2016) DISC1, astrocytes and neuronal maturation: a possible mechanistic link with implications for mental disorders. J Neurochem 138(4):518–524
Tanahashi S, Yamamura S, Nakagawa M, Motomura E, Okada M (2012) Clozapine, but not haloperidol, enhances glial D-serine and L-glutamate release in rat frontal cortex and primary cultured astrocytes. Br J Pharmacol 165:1543–1555
Oliveira da Cruz JF, Robin LM, Drago F, Marsicano G, Metna-Laurent M (2016) Astroglial type-1 cannabinoid receptor (CB1): a new player in the tripartite synapse. Neuroscience 323:35–42
Metna-Laurent M, Marsicano G (2015) Rising stars: modulation of brain functions by astroglial type-1 cannabinoid receptors. Glia 63(3):353–364
Navarrete M, Díez A, Araque A (2014) Astrocytes in endocannabinoid signalling. Philos Trans R Soc Lond B Biol Sci 369(1654):20130599
Suárez J et al (2010) Endocannabinoid system in the adult rat circumventricular areas: an immunohistochemical study. J Comp Neurol 518(15):3065–3085
Walter L, Franklin A, Witting A, Moller T, Stella N (2002) Astrocytes in culture produce anandamide and other acylethanolamides. J Biol Chem 277(23):20869–20876
Min R, Nevian T (2012) Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat Neurosci 15:746–753
Navarrete M, Araque A (2008) Endocannabinoids mediate neuron–astrocyte communication. Neuron 57:883–893
Navarrete M, Araque A (2010) Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68:113–126
Navarrete M et al (2013) Astrocyte calcium signal and gliotransmission in human brain tissue. Cereb Cortex 23:1240–1246
Han J et al (2012) Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell Mar 148(5):1039–1050
Shivachar AC (2007) Cannabinoids inhibit sodium-dependent, high-affinity excitatory amino acid transport in cultured rat cortical astrocytes. Biochem Pharmacol 73(12):2004–2011
Farina C, Aloisi F, Meinl E (2007) Astrocytes are active players in cerebral innate immunity. Trends Immunol 28:138–145
Aguirre-Rueda D et al (2015) WIN 55,212-2, agonist of cannabinoid receptors, prevents amyloid β1–42 effects on astrocytes in primary culture. PLoS One 10(4):e0122843
Froger N et al (2009) Cannabinoids prevent the opposite regulation of astroglial connexin43 hemichannels and gap junction channels induced by pro-inflammatory treatments. J Neurochem 111(6):1383–1397
Gajardo-Gómez R et al (2017) Cannabinoids prevent the amyloid β-induced activation of astroglial hemichannels: a neuroprotective mechanism. Glia 65(1):122–137
Molina-Holgado F, Molina-Holgado E, Guaza C, Rothwell NJ (2002) Role of CB1 and CB2 receptors in the inhibitory effects of cannabinoids on lipopolysaccharide-induced nitric oxide release in astrocyte cultures. J Neurosci Res 67:829–836
Ortega-Gutierrez S, Molina-Holgado E, Guaza C (2005) Effect of anandamide uptake inhibition in the production of nitric oxide and in the release of cytokines in astrocyte cultures. Glia 52:163–168
Sheng WS et al (2005) Synthetic cannabinoid WIN55,212-2 inhibits generation of inflammatory mediators by IL-1beta-stimulated human astrocytes. Glia 49:211–219
Esposito G et al (2011) Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS One 6(12):e28668
Rolland B et al (2013) Therapeutic prospects of PPARs in psychiatric disorders: a comprehensive review. Curr Drug Targets 14(7):724–732
Walter L, Dinh T, Stella N (2004) ATP induces a rapid and pronounced increase in 2-arachidonoylglycerol production by astrocytes, a response limited by monoacylglycerol lipase. J Neurosci 24:8068–8074
Grabner GF et al (2016) Deletion of monoglyceride lipase in astrocytes attenuates lipopolysaccharide-induced neuroinflammation. J Biol Chem 291(2):913–923
Viader A et al (2015) Metabolic interplay between astrocytes and neurons regulates endocannabinoid action. Cell Rep 12(5):798–808
Ignatowska-Jankowska et al (2014) In vivo characterization of the highly selective monoacylglycerol lipase inhibitor KML29: antinociceptive activity without cannabimimetic side effects. Br J Pharmacol 171:1392–1407
Pasquarelli N et al (2015) Neuropharmacology. Comparative biochemical characterization of the monoacylglycerol lipase inhibitor KML29 in brain, spinal cord, liver, spleen, fat and muscle tissue. Neuropharmacology 91:148–156
Howes OD, McCutcheon R (2017) Inflammation and the neural diathesis-stress hypothesis of schizophrenia: a reconceptualization. Transl Psychiatry 7(2):e1024. https://doi.org/10.1038/tp.2016.278
Pasternak O, Kubicki M, Shenton ME (2015) In vivo imaging of neuroinflammation in schizophrenia. Schizophr Res 41:85–93
Fernandes BS et al (2016) C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry 21(4):554–564
Kunz M et al (2011) Serum levels of IL-6, IL-10 and TNF-α in patients with bipolar disorder and schizophrenia: differences in pro- and anti-inflammatory balance. Rev Bras Psiquiatr 33(3):268–274
Watkins CC, Andrews SR (2016) Clinical studies of neuroinflammatory mechanisms in schizophrenia. Schizophr Res 176(1):14–22
Bayer TA, Buslei R, Havas L, Falkai P (1999) Evidence for activation of microglia in patients with psychiatric illnesses. Neurosci Lett 271:126–128
Bloomfield PS et al (2016) Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [C]PBR28 PET Brain Imaging Study. Am J Psychiatry 173:44–52
van Berckel BN et al (2008) Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry 64:820–822
Notter T et al (2017) Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol Psychiatry 23:323–334
Hafizi S et al (2017) Imaging microglial activation in untreated first-episode psychosis: a PET study with [18F]FEPPA. Am J Psychiatry 174(2):118–124
Mattei D et al (2014) Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav Immun 38:175–184
Müller N et al (2010) Celecoxib treatment in an early stage of schizophrenia: results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res 121(1–3):118 124
Chaudhry IB et al (2012) Minocycline benefits negative symptoms in early schizophre-nia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J Psychopharmacol 26:1185–1193
Kelly DL et al (2015) Adjunctive minocycline in clozapine-treated schizophrenia patients with persistent symptoms. J Clin Psychopharmacol 35:374–381
Fujita Y et al (2008) Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the antibiotic drug minocycline. Prog Neuropsychopharmacol Biol Psychiatry 32:336–339
Ribeiro BM et al (2013) Evidences for a progressive microglial activation and increase in iNOS expression in rats submitted to a neurodevelopmental model of schizophrenia: reversal by clozapine. Schizophr Res 151(1–3):12–19
Hu X et al (2012) Clozapine protects dopaminergic neurons from inflammation-induced damage by inhibiting microglial overactivation. J Neuroimmune Pharmacol 7(1):187–201
Cotel MC et al (2015) Microglial activation in the rat brain following chronic antipsychotic treatment at clinically relevant doses. Eur Neuropsychopharmacol 25(11):2098–2107
Ehrhart J et al (2005) Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J Neuroinflamm 2:29
Fernández-Ruiz J et al (2007) Cannabinoid CB2 receptor: a new target for the control of neural cell survival? Trends Pharmacol Sci 28:39–45
Mecha M et al (2015) Endocannabinoids drive the acquisition of an alternative phenotype in microglia. Brain Behav Immun 49:233–245
Mecha M, Carrillo-Salinas FJ, Feliú A, Mestre L, Guaza C (2016) Microglia activation states and cannabinoid system: therapeutic implications. Pharmacol Ther 166:40–55
Leweke FM (2012) Anandamide dysfunction in prodromal and established psychosis. Curr Pharm Des 18:5188–5193
Giuffrida A et al (2004) Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology 29:2108–2114
Koethe D et al (2009) Anandamide elevation in cerebrospinal fluid in initial prodromal states of psychosis. Br J Psychiatry 194:371–372
Seillier A, Advani T, Cassano T, Hensler JG, Giuffrida A (2010) Inhibition of fatty- acid amide hydrolase and CB1 receptor antagonism differentially affect behavioural responses in normal and PCP-treated rats. Int J Neuropsychopharmacol 13:373–386
Beltramo M et al (2000) Reversal of dopamine D(2) receptor responses by an anandamide transport inhibitor. J Neurosci 20:3401–3407
Amenta PS, Jallo JI, Tuma RF, Elliott MB (2012) A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J Neurosci Res 90(12):2293–2305
Walter L et al (2003) Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci 23:1398–1405
Malek N, Popiolek-Barczyk K, Mika J, Przewlocka B, Starowicz K (2015) Anandamide, acting via CB2 receptors, alleviates LPS-induced neuroinflammation in rat primary microglial cultures. Neural Plast 2015:130639
Aso E, Ferrer I (2016) CB2 cannabinoid receptor as potential target against Alzheimer’s disease. Front Neurosci 10:243
Martín-Moreno AM et al (2011) Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: relevance to Alzheimer’s disease. Mol Pharmacol 79(6):964–973
Cutando L et al (2013) Microglial activation underlies cerebellar deficits produced by repeated cannabis exposure. J Clin Invest 123(7):2816–2831
Gomes FV et al (2015) Decreased glial reactivity could be involved in the antipsychotic-like effect of cannabidiol. Schizophr Res 164(1–3):155–163
Kozela E et al (2010) Cannabinoids ∆9-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-κB and interferon-β/STAT proinflammatory pathways in BV-2 microglial cells. J Biol Chem 285:1616–1626
Juknat A, Kozela E, Kaushansky N, Mechoulam R, Vogel Z (2016) Anti-inflammatory effects of the cannabidiol derivative dimethylheptyl-cannabidiol—studies in BV-2 microglia and encephalitogenic T cells. J Basic Clin Physiol Pharmacol 27(3):289–296
Chew LJ, Fusar-Poli P, Schmitz T (2013) Oligodendroglial alterations and the role of microglia in white matter injury: relevance to schizophrenia. Dev Neurosci 35(2–3):102–129
Seki Y et al (2013) Pretreatment of aripiprazole and minocycline, but not haloperidol, suppresses oligodendrocyte damage from interferon-γ-stimulated microglia in co-culture model. Schizophr Res 151(1–3):20–28
Liddelow SA et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541(7638):481–487
Acknowledgements
This work was supported by Grant from the ‘Fundação de Amparo à Pesquisa do Estado de São Paulo’ (FAPESP Grant number 2013/08711-3 and 2014/10068-4), ‘Coordenação de Aperfeiçoamento de Pessoal de Nível Superior’ (Capes Grant number 1656470), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq Grant 460289/2014-4). DMS is also supported by Instituto Serrapilheira, Brazil (grant G-1709-16349).
Author information
Authors and Affiliations
Contributions
AV conceived the study, designed, and wrote the first draft and final version of manuscript. MSD conceived, supervised, and finalized the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
de Almeida, V., Martins-de-Souza, D. Cannabinoids and glial cells: possible mechanism to understand schizophrenia. Eur Arch Psychiatry Clin Neurosci 268, 727–737 (2018). https://doi.org/10.1007/s00406-018-0874-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-018-0874-6