Abstract
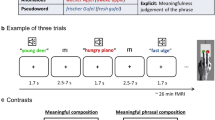
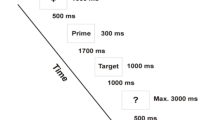
Patients with schizophrenia have semantic processing disturbances leading to expressive language deficits (formal thought disorder). The underlying pathology has been related to alterations in the semantic network and its neural correlates. Moreover, crossmodal processing, an important aspect of communication, is impaired in schizophrenia. Here we investigated specific processing abnormalities in patients with schizophrenia with regard to modality and semantic distance in a semantic priming paradigm. Fourteen patients with schizophrenia and fourteen demographically matched controls made visual lexical decisions on successively presented word-pairs (SOA = 350 ms) with direct or indirect relations, unrelated word-pairs, and pseudoword-target stimuli during fMRI measurement. Stimuli were presented in a unimodal (visual) or crossmodal (auditory-visual) fashion. On the neural level, the effect of semantic relation indicated differences (patients > controls) within the right angular gyrus and precuneus. The effect of modality revealed differences (controls > patients) within the left superior frontal, middle temporal, inferior occipital, right angular gyri, and anterior cingulate cortex. Semantic distance (direct vs. indirect) induced distinct activations within the left middle temporal, fusiform gyrus, right precuneus, and thalamus with patients showing fewer differences between direct and indirect word-pairs. The results highlight aberrant priming-related brain responses in patients with schizophrenia. Enhanced activation for patients possibly reflects deficits in semantic processes that might be caused by a delayed and enhanced spread of activation within the semantic network. Modality-specific decreases of activation in patients might be related to impaired perceptual integration. Those deficits could induce and increase the prominent symptoms of schizophrenia like impaired speech processing.



Similar content being viewed by others
Notes
The use of CPZ equivalents is a common practice to chart the relative antipsychotic potencies of antipsychotic drugs.
As one anonymous reviewer pointed out medication and illness duration might be considered as covariates for the analyses. However, an earlier meta-analysis showed that duration of illness had no effect on results of semantic priming [33]. However, we tried to keep our patient sample as homogeneous as possible via including only moderately ill patients. Medication status clearly influences neurocognitive functions but only patients that were on stable doses of atypical antipsychotic medication were included (e.g., risperidone, aripiprazole, quetiapine). In addition, earlier studies did not found any relation between medication and verbal memory in the treatment of schizophrenia [37].
We thank one anonymous reviewer for this suggestion.
References
Amodio DM, Frith CD (2006) Meeting of minds: the medial frontal cortex and social cognition. Nat Rev 7:268–277
Andreasen NC (1984a) The scale for the assessment of negative symptoms (sans). University of Iowa, Iowa City
Andreasen NC (1984b) The scale for the assessment of positive symptoms (saps). University of Iowa, Iowa City
Andreasen NC (1997) The role of the thalamus in schizophrenia. Can J Psychiatry 42:27–33
Assaf M, Calhoun VD, Kuzu CH, Kraut MA, Rivkin PR, Hart J Jr, Pearlson GD (2006) Neural correlates of the object-recall process in semantic memory. Psychiatry Res 147:115–126
Binder JR, Desai RH, Graves WW, Conant LL (2009) Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex 19:2767–2796
Copland DA, de Zubicaray GI, McMahon K, Eastburn M (2007) Neural correlates of semantic priming for ambiguous words: an event-related fMRI study. Brain Res 1131:163–172
David AS (1994) Dysmodularity: a neurocognitive model for schizophrenia. Schizophr Bull 20:249–255
de Gelder B, Vroomen J, Annen L, Masthof E, Hodiamont P (2002) Audio-visual integration in schizophrenia. Schizophr Res 59:211–218
de Gelder B, Vroomen J, de Jong SJ, Masthoff ED, Trompenaars FJ, Hodiamont P (2005) Multisensory integration of emotional faces and voices in schizophrenics. Schizophr Res 72:195–203
Docherty NM, Gordinier SW, Hall MJ, Dombrowski ME (2004) Referential communication disturbances in the speech of nonschizophrenic siblings of schizophrenia patients. J Abnorm Psychol 113:399–405
Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005) A new spm toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335
Fodor JA (1983) Modularity of mind: an essay on faculty psychology. MIT Press, Cambridge
Froud K, Titone D, Marantz A, Levy DL (2010) Brain/behavior asymmetry in schizophrenia: a meg study of cross-modal semantic priming. J Neurolinguistics 23:223–239
Han SD, Wible CG (2010) Neuroimaging of semantic processing in schizophrenia: a parametric priming approach. Int J Psychophysiol 75:100–106
Henson R (2003) Neuroimaging studies of priming. Prog Neurobiol 70:53–81
Jamadar S, O’Neil KM, Pearlson GD, Ansari M, Gill A, Jagannathan K, Assaf M (2013) Impairment in semantic retrieval is associated with symptoms in schizophrenia but not bipolar disorder. Biol Psychiatry 73:555–564
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (panss) for schizophrenia. Schizophr Bull 13:261–276
Kircher TT, Sass K, Sachs O, Krach S (2009) Priming words with pictures: neural correlates of semantic associations in a cross-modal priming task using fMRI. Hum Brain Mapp 30:4116–4128
Kubicki M, McCarley RW, Nestor PG, Huh T, Kikinis R, Shenton ME, Wible CG (2003) An fMRI study of semantic processing in men with schizophrenia. Neuroimage 20:1923–1933
Kuperberg GR, Deckersbach T, Holt DJ, Goff D, West WC (2007) Increased temporal and prefrontal activity in response to semantic associations in schizophrenia. Arch Gen Psychiatry 64:138–151
Kuperberg GR, Lakshmanan BM, Greve DN, West WC (2008) Task and semantic relationship influence both the polarity and localization of hemodynamic modulation during lexico-semantic processing. Hum Brain Mapp 29:544–561
Lehrl S (2005) Mehrfachwahl-wortschatz-intelligenztest mwt-b. Spitta Verlag, Balingen
Mathalon DH, Faustman WO, Ford JM (2002) N400 and automatic semantic processing abnormalities in patients with schizophrenia. Arch Gen Psychiatry 59:641–648
Minzenberg MJ, Ober BA, Vinogradov S (2002) Semantic priming in schizophrenia: a review and synthesis. J Int Neuropsychol Soc 8:699–720
Moritz S, Mersmann K, Kloss M, Jacobsen D, Wilke U, Andresen B, Naber D, Pawlik K (2001) ‘Hyper-priming’ in thought-disordered schizophrenic patients. Psychol Med 31:221–229
Neely JH (1991) Semantic priming effects in visual word recognition: a selective review of current findings and theories. In: Besner D, Humphreys GW (eds) Basic processes in reading: visual word recognition. Lawrence Erlbaum, Hillsdale (NJ), pp 264–336
Nestor PG, Valdman O, Niznikiewicz M, Spencer K, McCarley RW, Shenton ME (2006) Word priming in schizophrenia: associational and semantic influences. Schizophr Res 82:139–142
Niznikiewicz M, Mittal MS, Nestor PG, McCarley RW (2010) Abnormal inhibitory processes in semantic networks in schizophrenia. Int J Psychophysiol 75:133–140
Niznikiewicz MA, Friedman M, Shenton ME, Voglmaier M, Nestor PG, Frumin M, Seidman L, Sutton J, McCarley RW (2004) Processing sentence context in women with schizotypal personality disorder: an ERP study. Psychophysiology 41:367–371
Oldfield RC (1971) The assessment and analysis of handedness: the edinburgh inventory. Neuropsychologia 9:97–113
Pomarol-Clotet E, Oh TM, Laws KR, McKenna PJ (2008) Semantic priming in schizophrenia: systematic review and meta-analysis. Br J Psychiatry 192:92–97
Raettig T, Kotz SA (2008) Auditory processing of different types of pseudo-words: an event-related fMRI study. Neuroimage 39:1420–1428
Ragland JD, Gur RC, Raz J, Schroeder L, Kohler CG, Smith RJ, Alavi A, Gur RE (2001) Effect of schizophrenia on frontotemporal activity during word encoding and recognition: a pet cerebral blood flow study. Am J Psychiatry 158:1114–1125
Ragland JD, Moelter ST, Bhati MT, Valdez JN, Kohler CG, Siegel SJ, Gur RC, Gur RE (2008) Effect of retrieval effort and switching demand on fMRI activation during semantic word generation in schizophrenia. Schizophr Res 99:312–323
Rametti G, Junque C, Vendrell P, Catalan R, Penades R, Bargallo N, Bernardo M (2009) Hippocampal underactivation in an fMRI study of word and face memory recognition in schizophrenia. Eur Arch Psychiatry Clin Neurosci 259:203–211
Raposo A, Moss HE, Stamatakis EA, Tyler LK (2006) Repetition suppression and semantic enhancement: an investigation of the neural correlates of priming. Neuropsychologia 44:2284–2295
Ratcliff R (1993) Methods for dealing with reaction time outliers. Psychol Bull 114:510–532
Ross LA, Saint-Amour D, Leavitt VM, Molholm S, Javitt DC, Foxe JJ (2007) Impaired multisensory processing in schizophrenia: deficits in the visual enhancement of speech comprehension under noisy environmental conditions. Schizophr Res 97:173–183
Rossell SL, Price CJ, Nobre AC (2003) The anatomy and time course of semantic priming investigated by fMRI and ERPs. Neuropsychologia 41:550–564
Sass K, Krach S, Sachs O, Kircher T (2009) Lion–tiger–stripes: neural correlates of indirect semantic priming across processing modalities. Neuroimage 45:224–236
Schmitt A, Hasan A, Gruber O, Falkai P (2011) Schizophrenia as a disorder of disconnectivity. Eur Arch Psychiatry Clin Neurosci 261(Suppl 2):S150–S154
Seubert J, Loughead J, Kellermann T, Boers F, Brensinger CM, Habel U (2010) Multisensory integration of emotionally valenced olfactory-visual information in patients with schizophrenia and healthy controls. J Psychiatry Neurosci 35:185–194
Slotnick SD (2003) Model fitting in (n+1) dimensions. Behav Res Methods Instrum Comput 35:322–324
Spitzer M, Braun U, Hermle L, Maier S (1993) Associative semantic network dysfunction in thought-disordered schizophrenic patients: direct evidence from indirect semantic priming. Biol Psychiatry 34:864–877
Spitzer M, Braun U, Maier S, Hermle L, Maher BA (1993) Indirect semantic priming in schizophrenic patients. Schizophr Res 11:71–80
Straube B, Green A, Sass K, Kirner-Veselinovic A, Kircher T (2012) Neural integration of speech and gesture in schizophrenia: evidence for differential processing of metaphoric gestures. Hum Brain Mapp 34:1696–1712
Surguladze S, Rossell S, Rabe-Hesketh S, David AS (2002) Cross-modal semantic priming in schizophrenia. J Int Neuropsychol Soc 8:884–892
Titone D, Holzman PS, Levy DL (2002) Idiom processing in schizophrenia: literal implausibility saves the day for idiom priming. J Abnorm Psychol 111:313–320
Wible CG, Han SD, Spencer MH, Kubicki M, Niznikiewicz MH, Jolesz FA, McCarley RW, Nestor P (2006) Connectivity among semantic associates: an fMRI study of semantic priming. Brain Lang 97:294–305
Wiggs CL, Martin A (1998) Properties and mechanisms of perceptual priming. Curr Opin Neurobiol 8:227–233
Williams LE, Light GA, Braff DL, Ramachandran VS (2010) Reduced multisensory integration in patients with schizophrenia on a target detection task. Neuropsychologia 48:3128–3136
Wittchen H-U, Zaudig M, Fydrich T (1997) Skid—strukturiertes klinisches interview für dsm-iv. Hogrefe, Göttingen
Acknowledgments
We thank Georg Eder for fMRI data acquisition and Antonia Green for their support during the data collection as well as Franziska Kintzel for her help with data analyses. This work is supported by a grant from the Interdisciplinary Center for Clinical Research “BIOMAT” within the Faculty of Medicine at the RWTH Aachen University (IZKF VV N3) and by the International Research Training Group (IRTG 1328) of the German Research Foundation (DFG). KS funded by a grant from the German Research Foundation (DFG, SA 2221/3-1).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sass, K., Heim, S., Sachs, O. et al. Neural correlates of semantic associations in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci 264, 143–154 (2014). https://doi.org/10.1007/s00406-013-0425-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-013-0425-0